Article Text
Statistics from Altmetric.com
- rheumatoid arthritis
- etanercept
- infliximab
- adalimumab
- anakinra
- population study
- regional registry
- chronic arthritis
This study aimed at assessing the drug costs for biological treatments in a geographically defined area in southern Sweden (Scania province, population 1 145 090, November 2002), and at identifying any geographical differences and changes with time in the overall use of these compounds. Also, we wanted to investigate the completeness of the registry held by the South Swedish Arthritis Treatment Group (SSATG).1 During the study period no economic prescribing restrictions existed for these drugs in the region. The Swedish social security system covers all prescribed drug costs exceeding SEK 1800 (€170) a year to all patients in need, where need is based on their physician’s judgment. Thus, the use of biological antirheumatic treatment was limited only by restricted drug availability and capacity of the administration facilities. Medical practice was, however, under strong influence by guidelines from the Swedish Rheumatological Association.
SSATG data were used to explore annual and regional relations, assessing the current and previous use of biological agents, prescription costs, and patient’s diagnoses. The figures were adjusted according to the census population registry during 2000–03. Owing to legal constraints, which do not allow direct data linkage between SSATG and prescription databases, SSATG derived annual theoretical costs and sales of pharmaceutical agents to outpatients during the period 2000–03 (public domain) were broken down according to the patient’s district of residency as the unifying concept. To obtain an estimate of the costs per week of using the different drugs we assumed the annual drug dosage to be 2550 mg etanercept, 2100 mg infliximab, 1040 mg adalimumab, and 36 500 mg anakinra, respectively. Year-specific pharmacy unit drug costs were used. In 2002 the resulting costs were SEK 144 935 (1 SEK = 0.1€) for etanercept, SEK 108 539 for infliximab, SEK 143 377 for anakinra, and SEK 112 895 for adalimumab. The estimated yearly consumption of etanercept and infliximab was based on a previous detailed health economic study,2 whereas for anakinra and adalimumab we used the dosage recommended by the manufacturers. Registry data was checked against an assumed disease prevalence of 0.5% of the adult population.3
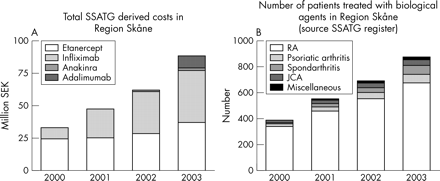
Costs per head varied by a factor of 10 between residential districts, mostly because of low population numbers in some residential districts. However, when related to the five larger healthcare districts, twofold differences remained. The proportion of patients treated increased progressively and was about 14.9% of all patients with rheumatoid arthritis (RA) in 2003. The proportion of diagnoses other than RA increased from 13.1% to 22.7% during the study (fig 1⇓). Pharmacy based and SSATG estimated cost ratios for all biological agents varied between 0.95 and 1.07 for the study years, but ratios for the individual drugs varied between 0.77 and 1.58. Concordance between pharmacy and SSATG cost figures increased with time (table 1⇓), mostly explained by the increasing number of rheumatological centres joining the SSATG. The number of biological treatments started increased from 24 to 46 per 100 000 inhabitants between 2000 and 2003 and the number of biological treatments withdrawn increased from 4 to 20 per 100 000 inhabitants. The proportion of new biological treatments in patients previously treated with a biological drug increased from 4% to 44% between 2000 and 2003.
Outward pharmaceutical sales and SSATG derived sales in SEK for biological drugs during the period 2000–03 in Scania (€1 = 9.05 SEK, $1 = SEK 8.5 SEK, May 2003)
Total and drug related yearly biological drug costs (A) and number of patients treated with biological drugs related to the diagnosis (B) during 1999–2003 in Scania, derived from SSATG information.
The regular overestimation of etanercept and underestimation of infliximab in SSATG costs suggests that the estimates have a systematic error. Several explanations can be offered, including annual pauses of longer than 1 week for etanercept, and prescription of infliximab for diagnoses other than those included in the SSATG registry. It can be estimated (table 1⇑) that the SSATG includes >90% of patients with arthritis currently treated with biological agents. This allows reliable continuous documentation of effects and potential side effects. Furthermore, a regional pharmacovigilance registry is also useful for assessing local differences in the use of the drugs and for evaluation of monitoring costs.
Acknowledgments
This study was supported by grants from Österlund and Kock Foundations, King Gustav V 80 year fund, and Reumatikerförbundet.