Article Text
Abstract
Background Cardiovascular (CV) risk stratification for patients with rheumatoid arthritis (RA) should facilitate evidence-based management. Prior work has derived an internally validated a CV risk score, the Expanded Cardiovascular Risk Prediction Score for Rheumatoid Arthritis (ERS-RA), using US data. The aim of this study was to perform an external validation among unselected patients with RA from Europe.
Methods Three large, partially overlapping, cohorts of patients with RA from the Swedish Rheumatology Quality register were identified for external validation, two with information on smoking and two with close to 10 years of median follow-up. The 10 -year rate of first CV events was assessed using the Kaplan-Meier method. The performance of ERS-RA was assessed using C-index and comparisons of observed versus predicted risks.
Results The C-index for ERS-RA varied across the three RA cohorts, from 0.75 to 0.78. Predicted risks corresponded well to observed risks among individuals with ≤10 % observed 10- year CV risk, but underestimated risk in individuals with a higher observed risk. In the absence of data on smoking, ERS-RA underestimated the CV risk by 3.3%, whereas in the cohorts including data on smoking, the calibration was within 1% (0.06% and 0.7%). In the clinically relevant risk intervals (<5%, 5.0%–<7.5%, 7.5%–<10%), ERS-RA performed well.
Conclusions In an unselected Swedish population with RA, ERS-RA performed well, although the 10-year CV risk was underestimated in high-risk groups and in the absence of data on smoking. ERS-RA could be considered as a risk stratification tool for targeted preventive interventions in clinical rheumatology practice.
- rheumatoid arthritis
- cardiovascular risk
- risk prediction
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0
Statistics from Altmetric.com
Key messages
What is already known about this subject?
Risk prediction tools developed for the general population tend to underestimate the risk of cardiovascular (CV) disease in patients with rheumatoid arthritis (RA).
The Expanded Cardiovascular Risk Prediction Score for Rheumatoid Arthritis (ERS-RA) was derived in the US Corrona RA registry and estimates the 10-year CV risk using dichotomous clinical variables and includes variables on RA disease severity and activity.
What does this study add?
In a Swedish population with RA, ERS-RA performed well in identifying patients with a low and high 10-year risk of CV event.
How might this impact on clinical practice?
In clinical routine practice, ERS-RA could be used to identify individuals with increased risk, who might be considered for preventive interventions, additional investigations or follow-up.
Introduction
Patients with rheumatoid arthritis (RA) are at increased risk of cardiovascular (CV) disease.1 2 Reports have described up to a 2-fold risk of acute coronary syndrome, including myocardial infarction and unstable angina, among patients with RA compared with the general population,3–8 with no or limited signs of improvement during recent years.4 9 10 Identifying individuals at high CV risk, and prevention of CV events, is therefore a major goal for rheumatology and for patients with RA. Studies aiming to evaluate the CV risk increase in RA point to a role for RA disease activity, which may potentiate the impact of traditional CV risk factors.11–16
Clinical risk prediction tools, such as the Systematic Coronary Risk Evaluation (SCORE), Framingham Risk Score, Reynolds Risk Score, the Pooled Cohort equations and QRISK2, all seek to estimate an individual’s absolute 10-year risk for CV events and play a prominent role in CV disease prevention in the general population.17–22 Among patients with RA, however, these tools perform suboptimally.23–26 Moreover, each of these CV risk prediction tools are based on measured values (eg, blood pressure, body mass index), or plasma concentrations (eg, lipids), of several risk factors.17–22 In a busy everyday rheumatology practice, information on the presence of these CV risk factors may be available, but actual values to enter into a risk score may not be as easily accessible.
The Expanded Cardiovascular Risk Prediction Score for Rheumatoid Arthritis (ERS-RA) provides an estimate of the 10-year risk of experiencing a myocardial infarction, stroke or CV death.27 In contrast to the above-mentioned scores intended for the general population, the ERS-RA contains several RA-specific variables. In addition, and again in contrast to most other CV risk scores, the CV risk variables used in the ERS-RA are included as dichotomous variables noted as present or absent, facilitating use in a rheumatology practice.
If validated in external cohorts, the ERS-RA might thus represent a useful tool to identify individuals with RA who are at high risk of CV disease and should receive lifestyle counselling and pharmacological treatment.28–31 The aim of this study was to perform an external validation of the ERS-RA in a large cohort of unselected patients with RA.
Methods
Setting
The Swedish Rheumatology Quality Register (SRQ) was started in 1995 and comprises data from patients with RA according to the 1987 ACR classification criteria.32 All public and private rheumatology departments report to the register. Estimates suggest that the SRQ covers >85% of all individuals diagnosed with RA in Sweden (personal communication, Daniela di Giuseppe, SRQ). By the use of the Swedish personal identification numbers, register data can be linked to mandatory public registers.
The National Patient Register includes dates and International Classification of Diseases (ICD)-codes for inpatient diagnoses since the 1960s, and since 2001 also outpatient diagnoses (except primary care). The Prescribed drug register holds data from prescribed drugs dispensed at public or private pharmacies since July 2005. The Cause-of-death Register, which started in 1961, includes dates and ICD-codes of underlying cause of death for all registered inhabitants who died in Sweden. Immigration and emigration dates are provided in the Population Register.33
In SRQ, RA-specific information (eg, erythrocyte sedimentation rate, C reactive protein, patient’s global and pain visual analogue scales (VAS), 28-joint count, disease activity score for 28-joint count (DAS28), disease activity (0–4) estimated by the physician and health assessment questionnaire (HAQ)), the date for RA disease symptom onset and information on pharmacological treatment (start and stop dates, name of drugs, dosage and interval) has been included since the start.34 35 Patient-reported smoking habits were added in 2011.
Study design and patient population
To maximise precision, the use of available information on smoking and the follow-up data, we defined and analysed three RA cohorts, presented in table 1.
The first RA cohort comprised patients in SRQ with RA between the years 2006 and 2011 (n=20 822). The start year 2006 was chosen to allow for identification of dispensed drugs from the Prescribed drug register. The first registered visit with disease activity and disability data in SRQ and after the cohort start date was used as the index visit, providing data on disease activity and pharmacological treatment. Only patients with a visit with a complete registration of HAQ, DAS28, and physician’s estimated disease activity were included. This cohort maximised precision, offered a comparatively long median follow-up, but included no data on smoking. Instead, the ERS-RA variable ‘current tobacco use’ was set to ‘no’ for all individuals. As smoking is a strong predictor of CV risk in ERS-RA, the risk of CV in these analyses was therefore likely to underestimate CV risks (in smokers).
The second RA cohort was a subgroup of the first RA cohort, defined as those patients who were also included in the EIRA population-based case-control study of incident RA (n=2047). The EIRA study has previously been described.36 37 Data on smoking habits had been collected at the inclusion in EIRA, which occurred at a mean of 3 years before index date in our study. (To verify that the second RA cohort did not otherwise differ from the larger first RA cohort in terms of CV risks, we also performed analyses in the second RA cohort where ‘current tobacco use’ was set to ‘no’ for all individuals.) This cohort thus included data on smoking, had a lower precision and a shorter median follow-up.
The third RA cohort was partially a subset of the first RA cohort, but comprised patients with a visit registered in SRQ during the time period from 1 January 2012 to 31 December 2015, when data on smoking status had been introduced in SRQ. In this cohort, the first visit after 1 January 2012 with data on HAQ, DAS28 and smoking status was used as the index visit. This cohort preserved precision, included data on smoking, but had a shorter yet more contemporary median follow-up time. Among the patients who previously had contributed person-time to cohort I, 10 073 patients were still at risk during the inclusion period for cohort III and were included in cohort III. Among those, 1172 had contributed person-time to cohort II as well. The mean time interval between the index visits in cohort I (and II) and cohort III was 4.8±2.2 years (median 5.0 years).
In all cohorts, patients with a myocardial infarction or stroke antedating the index date were excluded. To avoid reverse causation, that is, that data from the visit could be influenced by the CV disease, we also excluded patients with an event (myocardial infarction, stroke or CV death; ICD-codes in online supplementary table 1) occurring within 30 days from the index visit. No patients were excluded due to age; all patients 18 years or older at index date were included.
Supplemental material
Basic descriptive data of the included cohorts
Data on CV risk factors other than smoking
Information on age (at index date) and sex (male/female) was retrieved from SRQ. Presence of CV risk factors at index date, diabetes mellitus, hypertension and hyperlipidaemia, were identified by ICD-codes in The National Patient Register and/or Anatomical Therapeutic Chemical Classification (ATC)-codes in the Prescribed Drug Register (online supplementary table 1).
In the ERS-RA, moderate or high RA disease activity is measured as clinical disease activity index (CDAI)>10.38 As SRQ did not contain any measure of evaluator global disease activity on VAS 0–10.0 cm, two analyses, with alternative disease activity measurements, were performed in RA cohort I : (1) moderate or high RA disease activity was identified by DAS28 ≥3.2 and (2) moderate or high disease activity was identified by (a modified) CDAI >10 calculated using the physician’s estimation of the disease activity expressed on a Likert scale (0=No, 1=Mild, 2=Moderate, 3=Severe or 4=Maximal disease activity) times 2.5. In RA cohorts II and III, moderate or high disease activity was assessed by DAS28. For HAQ (extracted from SRQ), the cut-off >0.5 (present/absent) was used as in the original ERS-RA. Current prednisone use (present/absent) was identified as any oral glucocorticoid treatment registered at the index visit or as a dispensed prescription of an oral glucocorticoid in the Prescribed drug register (ATC-codes in online supplementary table 2) within 6 months before the index date. The registered date for RA symptom onset (in SRQ) was used to calculate the RA disease duration, which was categorised as ≥10 years or <10 years, as in ERS-RA.
Cardiovascular outcomes and follow-up
The outcome was defined as the first CV event retrieved from an inpatient diagnosis of myocardial infarction or stroke identified in The National Patient Register or death with CV disease (acute coronary syndrome, stroke, sudden cardiac death, congestive heart disease, arrhythmia or cardiogenic chock) as the underlying cause in the Cause-of-death register (ICD-codes in online supplementary table 1). All patients were followed up to 10 years after index date, their first event, emigration, death or 31 December 2015, whichever happened first.
Statistical methods
The baseline characteristics are presented as number and percentage or mean and SD. Data retrieved at the index date were used to calculate the 10-year risk of a CV event according to ERS-RA (online supplementary table 3). The 10-year CV event incidence rate was obtained using Kaplan-Meier survival curves. In RA cohort III with a limited time of follow-up, the observed 10-year CV risk was extrapolated using the method previously described.39 The discriminative ability was assessed using the overall C index.40 The calibration was calculated as the difference between the observed 10-year risk and the mean predicted 10-year risk in each cohort. Calibration plots were used to compare the predicted and observed CV risk in deciles of predicted risk. In clinical guidelines for primary prevention, statin initiation has been recommended for individuals with an estimated 10-year CV event risk of ≥10% or ≥7.5%.28–30 Although the underlying risk prediction models differ slightly, we chose to evaluate the calibration of ERS-RA in four clinically relevant intervals of 10-year CV risks:<5%, 5.0%–<7.5%, 7.5%–<10% and ≥10.0%. All analyses were performed using SAS for Windows, V.9.4 (SAS Institute, Cary, North Carolina, USA).
Results
Cohort characteristics
Characteristics of the three RA cohorts are presented in table 2. In the first RA cohort and during a median follow-up 7.2 years (total follow-up 144 475 person-years, 5687 patients (27.3%) had >9 years of follow-up), 2017 individuals experienced a first CV event (table 1). In the second RA cohort, median follow-up was 8.2 years, during which 136 CV events occurred (table 1). In the third RA cohort, median follow-up was 2.7 years, and 427 CV events occurred (table 1). The Kaplan-Meier adjusted observed 10-year CV incidence varied from 8.7% (RA cohort II) to 13.5% (RA cohort I). The proportions of myocardial infarction, stroke and CV death among the CV events were similar in the three cohorts (online supplementary table 4).
Characteristics at index date of included patients in the cohorts (n (%) unless otherwise noted)
Model performance
The C-index was similar in all three RA cohorts, and in analyses based on DAS28 as well as using CDAI; in RA cohort I using DAS28 as well as using CDAI, the C-index was 0.78 (95% CI 0.75 to 0.81); in RA cohort II including smoking status, it was 0.77 (95% CI 0.65 to 0.88); in RA cohort II disregarding smoking data, it was 0.75 (95% CI 0.62 to 0.86) and in RA cohort III, it was 0.76 (95% CI 0.69 to 0.82).
In the first RA cohort, ERS-RA underestimated the 10-year CV risk by 3.31% when the DAS28 was used and by 3.39% when the CDAI was used. The calibration plots showed that the estimated risks corresponded to the observed risks in the three lowest deciles of predicted risks, but that underestimation was observed in the four highest deciles (figure 1).
Calibration plots comparing the observed 10-year cardiovascular event rate with 95% CI and the mean risk of cardiovascular event in deciles predicted by ERS-RA using DAS28 and CDAI, respectively, in RA cohort I comprising patients with RA followed 2006 through 2011 with ERS-RA calculated without data on smoking. CDAI, clinical disease activity index; DAS28, disease activity score 28-joint count; ERS-RA, Expanded Cardiovascular Risk Prediction Score for Rheumatoid Arthritis; RA, rheumatoid arthritis.
In the second RA cohort, analyses disregarding smoking data showed a similar underestimation of risk in the higher deciles as in the first RA cohort. When smoking was included, calibration overall was precise (0.1%) although an overestimation of the risk remained among individuals with the highest predicted risks (figure 2).
Calibration plots comparing the observed 10-year cardiovascular event rate with 95% CI and the mean risk of cardiovascular event in deciles predicted by ERS-RA in RA cohort II including smoking status, RA cohort II with smoking status set to ‘no’ and in RA cohort III comprising patients with RA included 2012–2015 with available smoking status. ERS-RA, Expanded Cardiovascular Risk Prediction Score for Rheumatoid Arthritis; RA, rheumatoid arthritis.
In the third RA cohort, there was a negligible overestimation of the CV risk by 0.7% (figure 2).
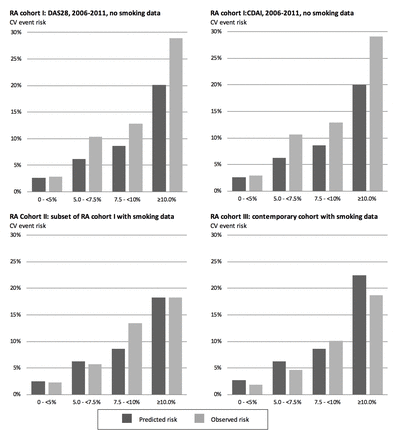
Table 3 and figure 3 describe calibration at risk levels of particular clinical interest (<5%, 5.0%–<7.5%, 7.5%–<10% and ≥10.0% 10-year CV event risk). The agreement between predicted and observed CV risk was within 1% in the group with the lowest predicted CV risk (<5%), which comprised 31%–48% of the patients in the cohorts (table 3). The first analyses (which did not include smoking) underestimated the CV risk by >4% in all other risk levels. In the analyses that included smoking status, the calibration was better. However, an underestimation by 1.5 in RA cohort II and 4.8% in RA cohort III was observed among individuals with an observed risk in the interval of 7.5%–<10%.
Observed and predicted 10-year cv risks, by clinical risk levels, in cohorts comprising patients with RA
Observed and predicted 10-year risks of CV events in the two analyses, using DAS28 and CDAI, respectively, of the RA cohort I, and in RA cohort II and III, presented by categories of predicted risks. CDAI, clinical disease activity index; CV, cardiovascular; DAS28, disease activity score 28-joint count; RA, rheumatoid arthritis.
Discussion
In this study, we assessed the external validity of the ERS-RA CV risk prediction algorithm in RA populations who were geographically and temporally distinct from the cohort used to derive the score. Overall, we found the ERS-RA to have good discriminatory capability as evidenced by high C-indices. However, as expected, CV risk was underestimated in analyses that did not include data on smoking. The ERS-RA generally performed well in analyses aimed to identify patients with a CV risk high enough to merit targeted CV risk factor intervention, that is, a 10-year risk of 7.5% or higher. But, we observed a general tendency towards underestimation of CV risks in individuals whose CV risks had already well exceeded clinical thresholds for primary preventive interventions.
Estimating CV risk in RA entails a number of unique challenges. Patients with high disease activity have the highest CV risk, but systemic inflammation is associated with a decrease in the total cholesterol concentration.41 Low body weight in RA from cachexia is associated with a higher risk of CV death than obesity, and the increased body fat content in patients with RA may not be observed as abdominal obesity.42–44 This interplay between traditional and disease-related risk factors limits the usefulness of risk prediction algorithms based on data from the general population.23–26 These factors underpin the potential value of an RA-specific CV risk score, such as ERS-RA.
In the current external validation of ERS-RA using Swedish data, analyses that included data on smoking demonstrated a better discrimination than those without, which is expected as smoking is both an independent CV risk factor and associated with lack of response to RA therapy.45–47 To our knowledge, two previous external assessments of the ERS-RA have been performed: one in the international collaboration, ATACC-RA, and one in a regional Swedish early RA cohort.27 48 49 Similar to the analyses in ATACC-RA, we observed an overestimation of 10-year risk for the very highest risk decile(-s) in the analyses that included smoking data. The assessment performed in ATACC-RA used a smaller cohort comprising patients from three continents; this prior analysis also found an overestimation of 10-year risk in the highest risk deciles, but otherwise accurately classified risks.48The observed tendency of the ERS-RA to overestimate risks among high-risk patients has been observed in evaluations of other risk algorithms.50 51 As indicated above, this overestimation may have a limited impact on clinical decision-making, since patients would have truly exceeded the threshold for action.
In contrast, in the recently published study from a regional Swedish early RA cohort, no overestimation of risks was observed, and ERS-RA was observed to underestimate the risk in several strata.49 The cohort used in the early RA study differs from the cohort in the present study as well as from the derivation cohort; the index date was set at the time point for diagnosis and the patients are characterised by high disease activity and HAQ, but minimal disease duration. Patients were included from 1995 to 2009, a period during which the incidence of CV disease decreased considerably, both among patients with RA and in the general population. This might, together with the limited number of patients (n=665, total follow-up not reported) and events (n=73) in the cohort, explain the observed inconsistency in risk differences between strata, and the underperformance compared with the present study.
There is no single obvious threshold of CV risk for the recommendation of CV prevention. Recommendations vary depending on indices used and which type of intervention is indicated based on the risk score; for example, smoking cessation is recommended to all individuals regardless of CV risk.28–31 The American College of Cardiology/American Heart Association ACC/AHA) recommends moderate-intensity or high-intensity statin therapy for patients with ≥7.5% 10-year CV risk estimated by the Pooled Cohort Equations, with similar outcomes as the ERS-RA.30 For patients with 5%–<7.5% 10-year CV risk the guidelines recommend moderate-intensity statin to be considered.30 In the evaluation of 10-year CV risks in these clinically important intervals for primary prevention (5%–10%), ERS-RA showed an excellent calibration, at least when data on smoking were included.
One question which we were unable to address in this evaluation, is the prevalence of unobserved traditional CV risk factors. Although regular CV risk screening is recommended in RA as well as in the general population,20 31 52 a recent study showed that hyperlipidaemia and hypertension often were unrecognised in patients with RA.53 Unobserved traditional CV risk factors among patients with increased CV risk would cause all available CV risk scores (including ERS-RA) to underestimate the CV risk as the (de facto) presence of these risk factors would be overlooked when scoring the individual patient. It cannot be ruled out that unobserved traditional risk factors influenced the underestimation of risks that was observed in some risk intervals in this study.
A limitation of the present study was the lack of smoking data in the cohort with the longest follow-up time. To provide more precise estimates of risk estimation with smoking data, two RA cohorts with smoking data were included. The RA cohorts with smoking data showed better overall calibration, but the more recent RA cohort III describes the inherent challenge for any evaluation of CV risk scoring based on 10-year risks with short available follow-up in any contemporary population. None of the cohorts supplied any laboratory data on traditional CV risk factors, which also shows the difficulty with scores reliant on such data in clinical practice. The ERS-RA relies on the presence or absence of a given risk factor, not actual laboratory values, facilitating its use in routine practice.
The main strength of this study is the large, nationwide, population-based RA cohort, with the linkage of prospectively collected data from mandatory public registers. The risk of misclassification of the outcomes is low.54 55 The present coverage of the SRQ is high (>85% for the year 2016), but some degree of selection is possible as individuals with severe comorbidity and expected short survival might not be included in any longitudinal clinical monitoring system.
It is important to identify patients with an increased CV risk, in whom clinicians should initiate interventions aiming for CV prevention. In a clinical context, such as rheumatological practice, detailed information on CV risk factors may be scarce. Similar to FRAX being a useful tool for estimating risk and guide further risk characterisation or institution of preventive measures in clinical practice,56 ERS-RA might be a useful tool to accurately identify individuals at low,<5%, and high, ≥10%, 10-year risk; those at high risk might be considered for a more intensive preventive measures, such as lipid lowering treatments. Patients with 10-year risks around 7.5% may be subject to preventive interventions, additional investigations or follow-up, after clinical considerations. Also, strategies guided by the ERS-RA might be considered for testing in randomised trials of CV prevention.
Acknowledgments
We would like to thank professor Lars Klareskog and professor Lars Alfredsson, Karolinska INstitutet, Stockholm for the use of EIRA data in the sensitivity analyses. We are also grateful to all patients and colleagues in rheumatology in Sweden who have contributed data to the Swedish Rheumatology Quality Register.
References
Footnotes
Contributors LL had full access to all of the data used for analyses in this study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. All authors are justifiably credited with authorship, according to the authorship criteria. Study concept and design: LL, PU, KPL, DHS, JA. Acquisition of data: JA. Statistical analysis: LL, PU, JA. Analysis and interpretation of data: LL, PU, KPL, JDG, CJE, DHS. Drafting of manuscript: LL, PU, JA. Critical revision of manuscript and final approval given: LL, PU, KPL, JDG, CJE, DHS. Obtained funding: JA. Study supervision: DHS, JA.
Funding Västerbottens Läns Landsting (ALF), the Swedish Rheumatism Association, the Heart Foundation of Northern Sweden, the Swedish Society of Medicine, King Gustaf V's 80-year foundation. KPL is supported by the NIH R01 HL127118.
Disclaimer Funders had no impact on the design or interpretation of the study or its results.
Competing interests LL has received personal fees for educational activities by Pfizer. JA has or has had research agreements with Abbvie, BMS, MS, Pfizer, Roche, Astra-Zeneca, Lilly and UCB, mainly in the context of safety monitoring of biologics via ARTIS/The Swedish Biologics Register. DHS receives salary support through his hospital from unrelated research agreements with Amgen, Abbvie, Pfizer, Bristol Myers Squibb, Genentech and Corrona.
Patient consent Not required.
Ethics approval The Ethical Committee in Stockholm, Sweden.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Access to the national register data used for this study is granted on a restrictive basis and may not be shared without additional specific permissions from the Swedish register-holding authorities.