Article Text
Abstract
Objective Earlier detection of pulmonary arterial hypertension (PAH), a leading cause of death in systemic sclerosis (SSc), facilitates earlier treatment. The objective of this study was to develop the first evidence-based detection algorithm for PAH in SSc.
Methods In this cross-sectional, international study conducted in 62 experienced centres from North America, Europe and Asia, adults with SSc at increased risk of PAH (SSc for >3 years and predicted pulmonary diffusing capacity for carbon monoxide <60%) underwent a broad panel of non-invasive assessments followed by diagnostic right heart catheterisation (RHC). Univariable and multivariable analyses selected the best discriminatory variables for identifying PAH. After assessment for clinical plausibility and feasibility, these were incorporated into a two-step, internally validated detection algorithm. Nomograms for clinical practice use were developed.
Results Of 466 SSc patients at increased risk of PAH, 87 (19%) had RHC-confirmed PAH. PAH was mild (64% in WHO functional class I/II). Six simple assessments in Step 1 of the algorithm determined referral to echocardiography. In Step 2, the Step 1 prediction score and two echocardiographic variables determined referral to RHC. The DETECT algorithm recommended RHC in 62% of patients (referral rate) and missed 4% of PAH patients (false negatives). By comparison, applying European Society of Cardiology/European Respiratory Society guidelines to these patients, 29% of diagnoses were missed while requiring an RHC referral rate of 40%.
Conclusions The novel, evidence-based DETECT algorithm for PAH detection in SSc is a sensitive, non-invasive tool which minimises missed diagnoses, identifies milder disease and addresses resource usage.
- Systemic Sclerosis
- Arterial Hypertension
- Epidemiology
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
Statistics from Altmetric.com
Introduction
The diagnosis of pulmonary arterial hypertension (PAH) is defined at right heart catheterisation (RHC) by a mean pulmonary arterial pressure (mPAP) of ≥25 mm Hg with a pulmonary capillary wedge pressure (PCWP) of ≤15 mm Hg.1 Additional diagnostic criteria may include a normal or reduced cardiac output1 or a pulmonary vascular resistance (PVR) of >3 Wood units.2 PAH includes diverse clinical phenotypes, prominent among which is systemic sclerosis (SSc, scleroderma) where PAH has emerged as a leading cause of death.3 ,4 Three-year survival for SSc patients with PAH has been estimated to be 56% compared with 94% in those without PAH.5 Observational studies have demonstrated that mortality remains high in SSc patients with PAH despite current best therapy.6 ,7
Poor outcome of PAH in SSc may be partially explained by disease-related comorbidities but also by delay in diagnosis. One recent study observed a better prognosis in subjects identified in an active screening programme compared with those identified in the course of routine practice,8 suggesting potential benefit of intervention earlier in the course of disease. This is consistent with the beneficial treatment effects demonstrated in early PAH.9 Current screening recommendations are largely based on consensus.1 ,2 Several organisations, including the American College of Cardiology Foundation/American Heart Association and the European Society of Cardiology/European Respiratory Society (ESC/ERS), have published a variety of screening recommendations relying mainly on symptoms and abnormal findings on transthoracic echocardiography.1 ,2 ,10 Other clinical tools include N-terminal probrain natriuretic peptide (NTproBNP) as a marker of myocardial stress,11 and disproportionately reduced pulmonary diffusing capacity for carbon monoxide (DLCO).12–14 The most widely used echocardiographic parameter, tricuspid regurgitant jet (TR) velocity, does not accurately reflect invasive pressures and is not present in all patients.15 ,16 Furthermore, while TR velocity recommendations are very specific in current guidelines, recommendations regarding other evidence of PAH (eg, symptoms) are less detailed; thus, application is likely to be variable between clinicians. More importantly, no previous screening studies have systematically performed RHC in all patients, precluding assessment of the rate of missed diagnoses (false negatives).
Our study provides evidence-based data guided by the principles of screening17: (A) employing rigorous methodology using the appropriate cross-sectional study design in order to determine the performance characteristics of the screening algorithm (sensitivity, specificity, etc); (B) evaluating accessible and feasible real-world screening tools; (C) identifying patients during an asymptomatic phase of disease (the SSc population in this study has symptoms which are not PAH specific) and (D) identifying patients for whom subsequent management is appropriate. Using the anchor of systematic RHC, the objective of the DETECT study was to develop a detection algorithm for PAH in SSc patients that would minimise the number of missed PAH diagnoses, while optimising the use of diagnostic RHC.
Methods
Study design
DETECT was designed as a cross-sectional study in which RHC and echocardiography were systematically conducted according to standardised procedures. Serum laboratory testing and data management were performed centrally, and data quality was rigorously monitored. DETECT was conducted in accordance with the Declaration of Helsinki and its amendments, followed the International Conference on Harmonisation Guideline for Good Clinical Practice, and was approved by local institutional review boards/ethics committees. All patients provided written informed consent.
Study population
Sixty-two experienced centres (managing at least 40 SSc patients) from 18 countries in North America, Europe and Asia participated in the study between 2008 and 2011. Patients aged ≥18 years with a diagnosis of SSc (American College of Rheumatology criteria,18 including patients with other connective tissue diseases who met these criteria) of >3 years’ duration from first non-Raynaud's symptom and a predicted DLCO of <60% (to enrich for a higher likelihood of PAH), were included. Patients were excluded if they had pulmonary hypertension (PH) confirmed by RHC prior to enrolment, were receiving recognised advanced PH therapy, had a forced vital capacity (FVC) <40% of predicted, renal insufficiency, previous evidence of clinically relevant left heart disease, or were pregnant.
Patients were classified as either non-PH, or WHO group 1 PH (PAH), WHO group 2 PH (PH due to left heart disease), or WHO group 3 PH (PH due to lung disease/hypoxia), according to current guidelines.10 ,19 WHO group 3 definition was based on Study Scientific Committee consensus applying a conservative interpretation of the literature to minimise misclassification of patients with evident lung disease as PAH. Classification definitions are summarised in figure 1.
Patient disposition. The results reported here focus on the 408 SSc patients with PAH (n=87) and those without PH (n=321; grey boxes). *One patient could not be assigned to a PH group due to a missing PCWP value. FVC, forced vital capacity; HRCT, high-resolution CT; mPAP, mean pulmonary arterial pressure; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; PCWP, pulmonary capillary wedge pressure; RHC, right heart catheterisation; SSc, systemic sclerosis.
Data collection and analysis
A broad range of variables potentially associated with PAH in SSc were assessed (112 in total; see online supplementary text). Four groups of variables were: (A) standard demographic and clinical parameters (68 variables in total, eg, SSc disease duration from first non-Raynaud's symptom, SSc subtype, SSc symptoms and organ involvement, general medical history, standard physical examination, 6-min walk distance, standard pulmonary function tests); (B) serum tests analysed by a central laboratory (antinuclear antibody profile (five antibodies), NTproBNP, endothelin-1, von Willebrand factor antigen, C-reactive protein, serum urate, creatinine, erythrocyte sedimentation rate, estimated glomerular filtration rate); (C) electrocardiography (ECG; right ventricular strain, right axis deviation, right bundle branch block) and (D) echocardiography according to standardised procedures (28 variables in total, eg, right atrium (RA) area, right ventricle (RV) area, RA diameter, TR velocity, tricuspid annular plane systolic excursion). To minimise bias, RHC as the confirmatory diagnostic test (conducted according to standardised procedures), was performed in all patients following collection of aforementioned data. Serious adverse events related to any study-mandated procedure (eg, RHC) were collected.
Statistical methodology
It was planned to enrol approximately 500 SSc patients including the planned number of 70 patients testing positive for PAH. This planned sample size took into consideration feasibility aspects of the study and assumed a prevalence rate of 14%.11 This sample size was calculated to allow an estimation of 90% sensitivity of the detection algorithm with a precision of ±7.5%. At a similar level of expected specificity, its precision is superior, due to the higher prevalence of non-PAH.
PAH and non-PH groups were described using summary statistics; sample size, mean, SD, median, upper and lower quartiles, minimum and maximum, and 95% CIs of the mean and median for quantitative data and frequencies (counts and percentages) for qualitative and categorical data.
Logistic regression modelling was the main analytical method, including linear and non-linear functional relations, where the binary outcome variable was PAH versus non-PH. Model-building entailed use of statistical procedures; variable selection was informed by clinical judgement and internal validation of models was performed via the bootstrap method.
Statistical analysis for selecting predictive variables and developing the detection algorithm for risk prediction of PAH was performed stepwise in three broad stages (see online supplementary text, tables S2–S5 and figures S1 and S2): (A) univariable and multivariable logistic regression models with RHC-based classification of PAH outcome, were applied within each of the four above-mentioned groups of candidate variables to select those associated with PAH; (B) the selected variables were further reduced across groups by using multivariable logistic regression; using nominal group technique, the Study Scientific Committee excluded some variables based on lack of clinical plausibility and/or feasibility with particular regard to resource limitations in standard practice and (C) a two-step decision tree was constructed based on two multivariable logistic regression models. The first step (sensitivity set at 97%) of the decision tree included non-echocardiographic tests to produce a risk prediction score that allowed exclusion of patients at low risk of having PAH and determined referral to echocardiography for the other patients. In the second step (specificity set at 35%), the risk score from Step 1 was combined with echocardiographic tests to produce the final PAH risk prediction score to determine if a patient should be referred to RHC for diagnosis. Spline functions with three knots were used in the models to adequately address non-linear relationships, which were initially identified by quadratic functions during the model-building process. Discriminatory performance to distinguish between PAH and non-PH patients was examined by receiver operating characteristic (ROC) curve analysis. The ROC area under the curve (AUC) formed the criterion for assessing the discriminatory ability of a model. Nomograms20 were derived from the two multivariable risk prediction models (see online supplementary text) to allow classification of patients into risk sets for referral to echocardiography (Step 1) and RHC (Step 2). An alternative algorithm with 65% specificity set in the second step was also evaluated, as was the application of the ESC/ERS guidelines to the DETECT population.1 The performance measures of the decision tree and its internal validation using bootstrap methodology are described in detail in the online supplementary text and tables S8–S11.
Results
Of 646 SSc patients screened, 158 did not meet the eligibility criteria (mostly due to DLCO values ≥60% of predicted). Of the 488 patients enrolled, 466 underwent RHC which revealed PH in 31% of patients (n=145) and PAH (WHO group 1 PH) in 19% (n=87; figure 1). Our results focus on these 87 PAH patients versus the 321 non-PH patients.
Patient characteristics, including RHC findings, are summarised in table 1.
Patient characteristics
In most patients (64%), PAH was mild (WHO functional class I or II), with moderately elevated mPAP and pulmonary vascular resistance and preserved mean cardiac index. In this population, among other variables, exercise capacity on 6-min walk test and dyspnoea were not associated with the presence of PAH. However, compared with non-PH patients, PAH patients were older, more likely to be male, in higher (more severe) WHO functional class, more likely to have the limited cutaneous form of SSc, to be anticentromere antibody (ACA) positive and to have a history of telangiectasias, had worse gas transfer (as assessed by DLCO), higher serum urate and NTproBNP levels, were more likely to have a right ventricular strain and right axis deviation on ECG, had larger RA and RV areas and higher TR velocity. However, when analysing commonly advocated TR velocity thresholds for PAH suspicion,1 ,21 20% of PAH patients were found to have a TR velocity of <2.5 m/s, 36% had a TR velocity of ≤2.8 m/s, and 63% had a TR velocity of ≤3.4 m/s (including 7% of PAH patients with undetectable TR velocity). Within the total DETECT cohort, 49% had a TR velocity of <2.5 m/s (including 13% with undetectable TR velocity). Several other echocardiographic variables (eg, tricuspid annular plane systolic excursion) were associated with the presence of PAH but did not progress to the final model (see below).
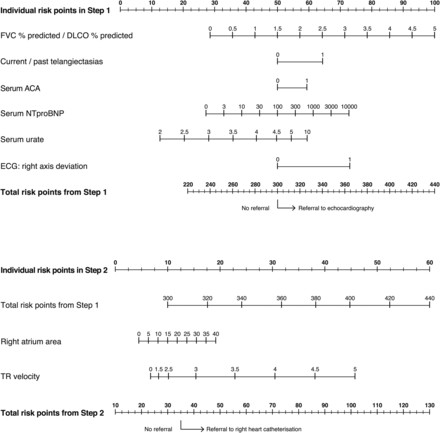
Following univariable and multivariable analyses, and clinical judgment of the Study Scientific Committee (based on feasibility and clinical plausibility), from an initial 112 variables, 13 were selected based on their discriminatory ability to detect PAH (see online supplementary text and table S3). These formed the basis for constructing a detection algorithm. To align the algorithm with real-world practice where the rheumatologist accesses non-echocardiographic data prior to referral to a cardiologist for echocardiography, the 13 variables were divided into nine non-echocardiographic variables (candidate variables in Step 1 of the algorithm) and four echocardiographic variables (candidate variables in Step 2). Subsequent multivariable analysis (stepwise forward procedure) resulted in six simple assessments being included in Step 1 of the algorithm to determine the need for referral to echocardiography (table 2). These were: FVC % predicted/DLCO % predicted, current/past telangiectasias, serum ACA, serum NTproBNP, serum urate and right axis deviation on ECG. Two echocardiographic variables (RA area and TR velocity) were included in Step 2 in order to determine the need for referral for RHC. Additionally, Step 2 included the carried-forward Step 1 risk prediction score (risk points) (table 2). Of all statistically selected final variables, the Study Scientific Committee replaced one echocardiographic variable (RV area) with another (RA area), because the latter is regarded as easier to assess and likely to be more reproducible. This replacement had a minimal effect on the performance of the Step 2 model (AUC 89% and 88% using RV area and RA area, respectively). The multivariable logistic regression models for the two-step decision tree are summarised in table 2.
Logistic regression models
Sensitivity (97% in Step 1) and specificity (35% in Step 2) were selected by the Study Scientific Committee with the aim of minimising the number of missed PAH diagnoses. The performance of the resulting DETECT algorithm is presented in figure 2, and a nomogram of the DETECT algorithm for use in clinical practice is shown in figure 3. Internal bootstrap validation generated consistent results and confirmed overall performance of the algorithm (see online supplementary text and tables S8 and S9). Exclusion of any single variable from the DETECT algorithm had only a small impact on model performance (see online supplementary text and table S11). If more than one variable is missing, the model cannot be used reliably; in clinical practice, a single missing variable should be handled as described in the legend to figure 3. Depending on the risk points in Step 1 of the algorithm, Step 2 (echocardiography) may not be needed for recommending RHC in some patients (figure 3).
Two-step decision tree for detection of pulmonary arterial hypertension in systemic sclerosis patients: the DETECT algorithm. Of the 408 SSc patients (87 PAH and 321 non-PH) at risk for PAH (SSc of >3 years’ duration, DLCO <60% of predicted, FVC ≥40% of predicted), data from 319 patients (72 PAH and 247 non-PH) were used for construction of the algorithm. All patients underwent right heart catheterisation. Sensitivity and specificity of the two steps of the algorithm (and the corresponding risk point cut-offs) were selected by the Study Scientific Committee with the aim of minimising the number of missed PAH diagnoses. Step 1: A complete dataset was available for 356 patients. The combined discriminatory ability of the six selected non-echocardiographic variables expressed as the AUC of the ROC curve was 84.4% (95% CI 79.5% to 89.8%) showing good discriminatory performance and no statistically significant lack of fit (see online supplementary appendix 5). At Step 1, a predefined sensitivity cut-off of 97% (corresponding to >300 risk points, compare figure 3), determined no referral to echocardiography in 52 patients. Among these, 50 were true negatives (patients without PAH on right heart catheterisation) and two were false negatives (PAH confirmed on right heart catheterisation). Step 2: A complete dataset was available for 267 patients. The AUC of the ROC curve for the total risk points from Step 1, plus the two selected echocardiographic variables, was 88.1% (95% CI 82.4% to 92.3%). A predefined specificity cut-off of 35% (corresponding to >35 risk points, compare figure 3), determined no referral to right heart catheterisation in 69 patients. Among these, 68 were true negatives and one was a false negative. Right heart catheterisation in the remaining 198 patients yielded 69 true positives (PAH confirmed) and 129 false positives. Thus, overall, the algorithm missed 3 (4%) out of the 72 PAH patients who had sufficient data to be included in the analysis. Note that the algorithm uses cut-offs for the risk points of the two steps only but not for individual parameters. ACA, anticentromere antibody; AUC, area under the curve; DLCO, pulmonary diffusing capacity for carbon monoxide; FVC, forced vital capacity; NTproBNP, N-terminal probrain natriuretic peptide; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; ROC, receiver operating characteristic; SSc, systemic sclerosis; TR, tricuspid regurgitant jet.
Nomograms for practical application of the DETECT algorithm: determination of the likelihood of pulmonary arterial hypertension and cut-off points for decision to refer a patient to echocardiography (Step 1) and subsequent right heart catheterisation (Step 2). At Step 1 (top panel), risk points for each of the six non-echocardiographic variables are calculated by reading from ‘Individual risk points in Step 1’ and adding them up to obtain a total. If the ‘Total risk points from Step 1’ is >300 (corresponding to a sensitivity of 97% as selected by the Study Scientific Committee) the patient is referred to echocardiography. Similarly, at Step 2 (bottom panel), risk points for the carried forward ‘Total risk points from Step 1’ and the two echocardiographic variables are calculated by reading from the ‘Individual risk points in Step 2’. If the ‘Total risk points from Step 2’ is >35 (corresponding to a specificity of 35% as selected by the Study Scientific Committee) the patient is referred to right heart catheterisation. Alternatively, being less conservative (65% predefined specificity at Step 2), the patient would be referred to right heart catheterisation if ‘Total risk points from Step 2’ is >40 (compare table 3 for the performance of these two options). Note that all variables will always contribute risk points irrespective of the measured value; for example, a negative serum ACA will contribute 50 risk points. Exclusion of any single variable from the DETECT algorithm has only a small impact on model performance (see online supplementary appendix 9). If a single Step 1 variable is missing it should be assigned 50 risk points, with the exception of current/past telangiectasias which should be assigned 65 points. If a single Step 2 variable is missing it should be assigned 10 points. The nomograms cannot be reliably used if more than one variable out of the eight total variables is missing. ACA, anticentromere antibody; DLCO, pulmonary diffusing capacity for carbon monoxide; FVC, forced vital capacity; NTproBNP, N-terminal probrain natriuretic peptide; TR, tricuspid regurgitant jet.
As presented in table 3, the rate of missed PAH diagnoses was 4% (n=3) applying the DETECT algorithm. The three missed PAH patients had mPAP values of 26, 25 and 30 mm Hg, PCWP values of 11–12 mm Hg, PVR values of 238–257 dyn·sec/cm5, TR velocities of 2.9, 2.5 and 2.6 m/s, RA areas of 16.4, 15.0 and 8.5 cm2, NTproBNP levels of 204, 47 and 71 pg/ml, no right axis deviation on ECG, and were ACA negative. The 4% missed diagnoses rate of the DETECT algorithm compares with 29% (n=24; see patient characteristics in online supplementary table S7) based on current ESC/ERS guidelines.1 The proportion of RHC which did not confirm a diagnosis of PAH was similar between the DETECT algorithm and the ESC/ERS guidelines (65% vs 60%). Applying the DETECT algorithm recommended referral of 62% of patients to RHC. Reducing the RHC referral rate from 62% to 41% (ie, to a similar level as the 40% RHC referral rate observed with the ESC/ERS guidelines) increased the rate of missed PAH diagnoses to 15% (n=11) which is still lower than the 29% achieved with the ESC/ERS guidelines (table 3).
Model performance: comparison of PAH detection approaches
Among the 466 patients who underwent RHC, one patient had a haematoma caused by accidental carotid puncture. This was managed without hospital admission or transfusion.
Discussion
DETECT was a large, multicentre, real-world, cross-sectional study with detailed population characterisation, standardised RHC and echocardiography procedures, central serum testing, central data management and rigorous data monitoring. It is the first PAH detection study to undertake systematic RHC in all patients and to develop an evidence-based algorithm using simple clinical data and non-invasive tests for earlier identification of PAH in a mildly symptomatic population. The DETECT study demonstrates that within this cohort of SSc patients, PAH is much more common than previous studies have suggested,21 ,22 and can be identified before symptoms are sufficiently advanced to be discriminated from general SSc symptoms.
Previous screening initiatives21 ,22 without systematic RHC in all patients, resulted in guideline recommendations based on symptoms and echocardiography without information on the number of missed PAH diagnoses (false negatives).1 ,2 ,10 Consequently, PAH continues to be diagnosed late with advanced symptoms.6 ,8 ,23 Given the evidence that early intervention may delay morbidity in PAH,9 and that screening programmes that allow earlier treatment in patients with PAH related to SSc may change the way patients are managed and may thus improve prognosis,8 ,24 the DETECT algorithm addresses a major medical need in this patient population. Among all patient populations at risk for developing PAH, SSc appears most suitable for screening programmes in terms of prevalence and feasibility.
To align the DETECT approach with clinical practice where the rheumatologist may have non-echocardiographic data available prior to referral to a cardiologist for echocardiography, a corresponding two-step algorithm was developed. This will limit echocardiography referral to patients at increased PAH risk. Of course, the algorithm can also be used if all data including echocardiography are available at the same time. Sensitivity and specificity of the two steps of the algorithm (and the corresponding risk point cut-offs) were selected by the Study Scientific Committee with the aim of minimising the number of missed PAH diagnoses as compared with the ESC/ERS guidelines1 (table 3). Given that the high overall sensitivity (96%) selected for the DETECT algorithm is associated with reduced specificity (48%), the rate of RHC required in the high-risk SSc population included in DETECT is substantial (62%). However, the proportion of RHC performed that did not confirm a diagnosis of PAH was similar between the DETECT algorithm and the ESC/ERS guidelines (65% vs 60%). Furthermore, overall RHC usage when applying the DETECT algorithm in clinical practice is likely to be lower than when compared with current guideline recommendations, since the latter are applied to the general SSc population, without enrichment for high PAH risk. For pragmatic reasons (sample size, reasonable expected positive predictive value) and ethical considerations (mandated RHC in all patients) the DETECT algorithm is designed for application in a high-risk SSc population (inclusion criteria DLCO <60% and SSc disease duration >3 years). The prevalence of PAH in SSc patients with a DLCO ≥60% is not known but may be as low as 1.2%, sevenfold lower than in patients with a DLCO <60%.21 Screening these patients would not reduce the rate of missed PAH diagnoses (4% in DETECT) much further, but would increase the rate of false positives; the number of RHCs needed per PAH diagnosis was three in DETECT, but would be six in a population with 10% prevalence and 11 in a population with 5% prevalence. In a regularly screened unselected SSc population, incidence and prevalence of PAH were found to increase progressively, with onset generally after 3 years of disease.25 Based on these data, it is likely that few PAH patients were missed in DETECT as a result of the required minimum SSc disease duration, and that this criterion contributed to selection of a high-risk population. It is possible, however, that some PAH patients with very early SSc and preserved DLCO were missed.
Adjusting the specificity of the DETECT algorithm to recommend similar RHC referral rates (in the DETECT high-risk population) as the ESC/ERS guidelines still substantially reduces the number of missed PAH diagnoses (table 3). As shown in this study and in previous published experience,26 RHC is a safe technique in experienced centres.
Clinical plausibility, feasibility and applicability of the final selected variables were assured by expert input, and their robustness was internally validated. Some parameters previously identified as predictive of PAH were confirmed in DETECT, such as FVC/DLCO,27 telangiectasias,27 ACA,27 ,28 NTproBNP,11 ,29 right axis deviation on ECG30 and TR velocity.8 ,22 Serum urate has not been described previously as being predictive of PAH, but was identified as such in this study where low values were associated with a low PAH risk. There is, however, some support for an association in the literature; in a study of 228 patients, serum urate levels were significantly higher in those with PAH than in age-matched controls,31 findings that have been corroborated elsewhere.32 Interestingly, we have demonstrated the limited utility of the two main components of current guidelines, that is, symptoms and echocardiography: dyspnoea, a prominent symptom of PAH, did not discriminate between PAH and absence of PH (which is consistent with its lack of sensitivity in identifying cardiopulmonary compromise in early disease perhaps due to SSc-associated restricted musculoskeletal mobility), and TR velocity alone would have missed 20% of PAH patients when using a PAH suspicion threshold of ≥2.5 m/s, 36% when using a threshold of >2.8 m/s and 63% when using a threshold of >3.4 m/s.
An expert consensus on criteria for referring SSc patients to RHC has been published recently.33 The objectives, methodology and population of this study were different from those of DETECT (consensus-based assessment of symptomatic patients with suspected PH rather than prospective data-driven assessment of a primarily screening population without requirement for PAH suspicion). Of the criteria proposed by the expert consensus, dyspnoea, physical findings related to the right heart and WHO functional class were not sufficiently predictive to proceed to the final DETECT algorithm. DLCO (and DLCO corrected for alveolar volume) was less predictive than FVC/DLCO. DETECT confirmed the value of TR velocity as one component of the algorithm despite poor performance as a single parameter. The predictive value of both RV and RA dilation was confirmed in DETECT; RA area is part of the final algorithm.
The inclusion criteria selected for prevalent SSc patients, which may have resulted in an over-representation of limited cutaneous SSc. Additionally, the DETECT algorithm was not developed to identify other forms of PH; application of the DETECT algorithm to the total PH population missed 19% of WHO group 2 PH patients, and 37% of WHO group 3 PH patients, both of which are common in SSc. The guideline definitions used for PH classification in DETECT do not consider other variables which may be relevant in clinical practice, for example, PVR, echocardiographic parameters to identify left ventricular disease, exercise haemodynamics or fluid challenge in borderline PH, or elevated transpulmonary gradient that may have increased risk of progression to PAH.34 Pulmonary veno-occlusive disease was not considered, since neither systematic radiological assessment nor lung biopsy were performed. Finally, the results are based on cross-sectional analyses; it is not possible to determine algorithm performance long-term, or to recommend how frequently patients should be assessed. Results from this study were not validated externally but internal validation using well-established methodology (bootstrapping) confirmed that our findings are robust.
In conclusion, in this cross-sectional multicentre study, we have addressed the fundamental flaw in all previous screening studies in PH by mandating diagnostic RHC in all patients and, thus, determining the false negative rate. The resultant DETECT algorithm is highly sensitive, reducing missed diagnoses when compared with the ESC/ERS guidelines and optimising resource usage by restricting detection efforts to the appropriate high-risk population. Evidence-based guideline recommendations for the identification of mildly symptomatic PAH patients can now be developed, facilitating earlier intervention.
Acknowledgments
The authors would like to thank all investigators and patients involved in the DETECT study. They would also like to thank Fabrice Kiefer, PhD and Aisha Rashid, BSc (Actelion Pharmaceuticals Ltd) and Outcome Europe SARL (St-Prex, Switzerland) for their operational support, Juan-Vicente Torres-Martin, MSc (Actelion Pharmaceuticals Ltd) for support of the statistical analyses and the creation of nomograms, Martin Schumacher, PhD (University of Freiburg, Germany) for statistical consultancy, and Julia Heagerty, PhD (Elements Communications Ltd, Westerham, UK) for medical writing support funded by Actelion Pharmaceuticals Ltd.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online supplement
Footnotes
-
Handling editor Tore K Kvien
-
JGC, CPD and EK contributed equally.
-
Contributors All authors except DK were involved in the study design. JGC, CPD, EG, DB, OD, DK, UM-L, JEP and MCV recruited patients to the study and collected data. HC-B and HH conducted the statistical analyses. DR provided epidemiology methods input. All authors had full access to the study data and were involved in the interpretation of results. JGC, JRS and MD drafted the manuscript which was critically reviewed and approved for submission by all authors.
-
Funding Actelion Pharmaceuticals Ltd funded the study and was responsible for designing the study protocol, data collection, and statistical analysis under the leadership of an independent Study Scientific Committee (non-Actelion authors, apart from HH).
-
Competing interests JGC has consultancy relationships, received lecture honoraria and/or has received research funding from Actelion Pharmaceuticals Ltd, Pfizer, GlaxoSmithKline and United Therapeutics. CPD has consultancy relationships, received lecture honoraria and/or has received research funding from Actelion Pharmaceuticals Ltd, Pfizer, GlaxoSmithKline, Sanofi-Aventis and Novartis. EG has received honoraria for consultations and/or speaking at conferences from Actelion Pharmaceuticals Ltd, Bayer, Gilead, GlaxoSmithKline, Lilly, Milteney, Novartis, Pfizer and Rotex Medica, and funding for clinical trials from Actelion Pharmaceuticals Ltd, Bayer, GlaxoSmithKline, Encysive, Lilly and Pfizer. DB has acted as a consultant for Actelion Pharmaceuticals Ltd. OD has consultancy relationships and/or has received research funding from Actelion Pharmaceuticals Ltd, Pfizer, Boehringer-Ingelheim, Bayer, Roche, Ergonex, Bristol-Myers Squibb, Sanofi-Aventis, United BioSource Corporation, Medac, Biovitrium, Novartis, 4D Science, Sinoxa and Active Biotec. He has received lecture honoraria from Actelion, Pfizer and Ergonex. DK has consultancy relationships, has served on speakers’ bureaus, and/or has received research funding from Actelion Pharmaceuticals Ltd, Bayer, Bristol-Myers Squibb, Gilead, Genentech, ISDIN, Merck, Roche, Sanofi-Aventis and United Therapeutics. UM-L has acted as a consultant and lecturer for Actelion Pharmaceuticals Ltd, Pfizer and GlaxoSmithKline. JEP has received payment for consultancy and lectures and received research funding from Amgen, Bristol-Myers Squibb and Pfizer. She has received payment for consultancy and lectures from Abbott, Janssen, UCB and Roche. She has received consultancy fees and research funding from Actelion Pharmaceuticals Ltd and research funding from Teva and Celgene. MCV has consultancy relationships, received lecture honoraria and/or has received research funding from Actelion Pharmaceuticals Ltd, Pfizer, GlaxoSmithKline, Therabel Pharma and United Therapeutics. MD, HC-B and DMR are full-time employees of Actelion Pharmaceuticals Ltd and have stock/stock options in the company. HH has acted as a consultant for Actelion Pharmaceuticals Ltd and has received research funding from Roche Austria. VVM has acted as a consultant and/or received honoraria/lecture fees from Actelion Pharmaceuticals Ltd, Bayer, Gilead and United Therapeutics. She has received research funding (to the University of Michigan) from Actelion Pharmaceuticals Ltd, Bayer, Novartis and United Therapeutics. JRS has consultancy relationships regarding development of therapies for cardiopulmonary complications of scleroderma with Actelion Pharmaceuticals Ltd, United Therapeutics, Pfizer, Gilead, Bayer, Boehringer-Ingelheim, Sigma Tau, FibroGen, Sanofi, Celgene, MedImmune, Genentech and Intermune. He has received research funding from Actelion Pharmaceuticals Ltd, United Therapeutics and Gilead Sciences and payment for speakers’ bureaus from United Therapeutics.
-
Provenance and peer review Not commissioned; externally peer reviewed.