Article Text
Abstract
Objective To assess the 6-month effectiveness of the first rituximab (RTX) course in rheumatoid arthritis (RA) and to identify possible predictors of response.
Method 10 European registries submitted anonymised datasets (baseline, 3- and 6-month follow-up) from patients with RA who had started RTX, and datasets were pooled and analysed. Heterogeneity between countries was analysed by analysis of variance. Predictors of response were identified by logistic regression.
Results 2019 patients were included (mean age/disease duration 53.8/12.1 years, 80.3% female, 85.6% rheumatoid factor (RF) positive and 76.8% (456/594 patients) anti-cyclic citrullinated peptide antibodies (anti-CCP) positive). For these patients an average of 2.7 disease-modifying antirheumatic drugs (DMARDs) (range 0–10) had failed, and RTX was given as the first biological agent in 36.6% of patients. There was significant heterogeneity between countries for several baseline characteristics, including the number of previous biological agents. Disease Activity Score based on 28 joint counts (DAS28) decreased from 5.8±1.4 at baseline to 4.2±1.4 at 6 months (p<0.0001) and 22.2%/42.5% achieved European League Against Rheumatism (EULAR) good/moderate response. Larger 6-month improvement in DAS28 was observed in RF-positive and anti-CCP-positive versus seronegative patients. The following predictors of EULAR good response at 6 months were identified in a multivariate analysis: anti-CCP positivity (OR=2.86, p=0.003), number of previous DMARDs (OR=0.84, p=0.06), ≤1 previous biological agents (OR=1.89, p=0.04), baseline DAS28 level (OR=0.74, p=0.003).
Conclusion In this large observational cohort of patients with RA treated with RTX, seropositive patients achieved significantly greater reductions in DAS28 at 6 months than seronegative patients. Effectiveness was best when RTX was used as the first biological agent or after failure of no more than one anti-tumour necrosis factor agent.
Statistics from Altmetric.com
Introduction
Rituximab (RTX) (Mabthera, Rituxan), a monoclonal antibody which selectively targets CD20-positive B cells, has been approved for the treatment of rheumatoid arthritis (RA) in many countries. With the standard approved dosage, a single course of two 1000 mg infusions given 2 weeks apart, a majority of patients in clinical trials exhibited an ACR20 (American College of Rheumatology 20% improvement) response and a similar proportion a European League Against Rheumatism (EULAR) moderate response.1 This beneficial effect has been demonstrated in patients naive to anti-tumour necrosis factor (anti-TNF) treatment as well as in patients for whom previous anti-TNF treatment has failed.1 2 Re-treatment after variable intervals of 6–12 months has been shown to be effective when a relapse occurred.3 4 Thus, when treatment with an anti-TNF agent has failed, RTX is now an approved and logical treatment option. Given the rapid evolution in the field of biological disease-modifying antirheumatic drugs (DMARDs), there are several treatment alternatives for patients for whom one TNF inhibitor has failed—for example, alternative TNF inhibitors, interleukin 1 and interleukin 6 inhibitors and a modulator of T cell costimulation.5,–,10 For this reason, a major challenge is to identify predictors of response, based on clinical, demographic, immunological or genetic data, for a more personalised treatment approach.11
Controlled clinical trials are the major source of information on efficacy and safety of drugs under experimental conditions. However, patients included in these studies do not always represent patients in clinical practice owing to strict inclusion and exclusion criteria. Furthermore, the more complex questions that govern clinical decision-making can rarely be considered adequately using randomised trials. In such instances longitudinal observational studies may provide useful information and more reliable answers to specific questions about the use of DMARDs in clinical practice. Real-life effectiveness data on RTX in RA may be particularly relevant, taking into account questions that have been raised about optimal dosing, co-medication and efficacy in seropositive versus seronegative patients.
During the past year we have initiated a European collaboration between registries from 10 different European countries, the European Collaborative Registries for the Evaluation of Rituximab in rheumatoid arthritis (CERERRA) initiative. The aim of this study is to present baseline characteristics of patients with RA treated with RTX, analyse 6-month effectiveness and identify possible predictors of response.
Patients and methods
The CERERRA is an investigator-led, industry-supported initiative aiming to evaluate clinical aspects of RTX use in patients with RA. This manuscript was prepared from the authors without any influence by the supporting medical industry. All 10 participating European registries (from the Czech Republic, Denmark, Finland, The Netherlands, Norway, Russia, Slovenia, Spain, Sweden and Switzerland) submitted fully anonymised datasets with baseline characteristics, including age, gender, disease duration, number of previous synthetic and biological DMARDs, rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibodies (anti-CCP) status of all patients with an established diagnosis of RA who had been treated with RTX.12,–,20 Levels of RF and anti-CCP were determined by local laboratories and local cut-off values for positivity were applied. Disease activity markers at baseline and after 3 and 6 months were also provided (number of swollen and tender joints, Visual Analogue Scales for pain, patient's and physician's global assessment, Disease Activity Score based on 28 joint counts (DAS28), Health Assessment Questionnaire) as well as information about concomitant drugs (DMARDs and glucocorticoids).
Statistical analysis
Appropriate parametric statistical tests were used for the analysis of data. All continuous data were examined for normal distribution by Kolmogorov–Smirnov test. Heterogeneity for baseline characteristics between countries was analysed by analysis of variance followed by post hoc test (Bonferroni/Dunn). Clinical response according to reductions in DAS28 and achievement of EULAR response criteria was analysed at 3 and 6 months. A second analysis was performed according to the number of previously used biological agents. Patients re-treated with RTX before 6 months were classified in the analyses as ‘non-responders’, regardless of their response according to the EULAR criteria.
Predictors of EULAR good response were identified by logistic regression analyses. We performed univariate logistic regression analyses adjusted for age and sex with EULAR good response at 6 months as the dependent variables and several demographic and disease variables considered to be clinically important as independent variables. The results from these analyses (p<0.25 as the criterion) and correlation analyses (Pearson and Spearman correlations) guided the selection of variables for the multivariate logistic regression analyses with dichotomised responses as the dependent variables. Age and sex were also included in the multivariate analyses. The non-significant variables were removed by stepwise backward selection. We then added back into the models, one at a time, any variable not originally selected from the univariate analyses. The variables were kept in the models if significant. Appropriate tests for linearity, interactions and goodness of fit were performed. Statistical analyses were done with StatView 5.0.1 for PC (SAS Institute, Cary, North Carolina, USA) and SPSS 15.0 for Windows.
Results
Baseline characteristics
The baseline characteristics for all patients are summarised in table 1 and shown according to country in online supplementary table S1. A total of 2019 patients were included with a mean (SD) age/disease duration of 53.8 (13.3)/12.1 (8.9) years. There was significant heterogeneity between the countries for age and disease duration. Patients in the Russian registry had the lowest mean age and shortest disease duration.
Baseline characteristics for all patients from the 10 European registries (Russia (Ru), Sweden (Swe), Norway (Nor), Finland (Fin), Denmark (Den), Slovenia (Slo), Spain (Sp), The Netherlands (Nth), the Czech Republic (Cz), Switzerland (Switz))
RF positivity was reported in 85.6% of the patients (table 1). Of the 594 patients with available data on anti-CCP status, 76.8% were positive. The numbers of confirmed double seropositive and double seronegative patients were 372 and 59, respectively. RF-positive patients were significantly older than RF-negative patients (mean (SD) age 54.9 (13.0) vs 50.3 (14.5), p<0.0001). No difference was found between the RF-positive and RF-negative subgroups for the number of previous DMARDs and biological agents, baseline DAS28, disease duration or concomitant DMARDs. Anti-CCP-positive patients had a significantly smaller number of previous biological DMARDs than anti-CCP-negative patients, but other differences in baseline characteristics were not observed between anti-CCP-positive and anti-CCP-negative patients.
The mean (SD) numbers of previous synthetic and biological DMARDs were 2.7 (1.6) and 1.1 (1.1), respectively. Patients in the Russian registry had used a significantly smaller number of previous biological DMARDs than patients from the other registries (online supplementary table S1).
Information about previous use of biological DMARDs was available in 1844 patients. For these patients two or more biological agents had previously failed in 596 (32.3%), one agent had failed in 574 (31.1%), and 674 (36.6%) patients received RTX as their first biological agent. The baseline characteristics stratified by number of previous biological agents are summarised in online supplementary table S2.
Concomitant synthetic DMARDs were used by 76.7% of the patients. The majority were co-treated with methotrexate (MTX) (64.8%), a small number received combination treatment with MTX and another synthetic DMARD, while a significant number of patients were treated with other DMARDs, such as sulfasalazine, azathioprine, ciclosporin, leflunomide, antimalarial agents and also gold salts in a few patients.
Treatment responses
The mean (SD) DAS28 at baseline was 5.8 (1.4) and decreased to 4.3 (1.3) and 4.2 (1.4) at 3- and 6-month assessments, respectively. The majority of patients (73.0%) had high baseline disease activity (DAS28>5.1) and 21.8% had moderate disease activity (DAS28 between 3.2 and 5.1). At 3 months the percentage of patients with high disease activity was markedly reduced and remained stable at the 6-month assessment (figure 1). A corresponding increase in the proportions of patients with low disease activity or in remission was seen (figure 1). EULAR good/moderate responses at 3 months were achieved by 195/483 out of 1087 patients (17.9%/44.4%) and by 210/402 out of 945 patients (22.2%/42.5%) after 6 months. DAS28 improvement of >1.2 was observed in 62.5% of patients with available ΔDAS28 at the 6-month follow-up (616/986 patients).
Levels of DAS28 at baseline, after 3 and 6 months.
The subset of RF-positive patients achieved significantly larger reductions in DAS28 than the patients who were RF negative (95% CI of the difference at 3 months −0.64 to −0.18, at 6 months −0.64 to −0.09) (table 2 and figure 2). Similar results were seen when anti-CCP positive and anti-CCP negative, as well as when double-positive versus double-negative patients were compared (table 2 and figure 2). The proportions of patients with EULAR good/moderate response are also depicted in table 2.
Mean ΔDAS28 (bars: SEM) during the first 6 months for seropositive and seronegative patients. At 3 and 6 months RF positive patients (A) as well as anti-CCP positive (B) and double positive patients (C) achieved significantly larger reductions of DAS28 compared to RF negative, anti-CCP negative and double negative patients, respectively.
Reductions in DAS28 at 3 and 6 months (mean (SD)) and achievement of EULAR good/moderate response (%) by RF and anti-CCP status
The majority of patients received a concomitant DMARD. At 3 months, responses were similar in patients with and without synthetic DMARD co-treatment, but after 6 months the mean improvement of DAS28 was significantly larger for patients receiving concomitant DMARDs (ΔDAS28 1.9 (1.5) vs 1.6 (1.5), p=0.04, 95% CI for the difference −0.47 to −0.03).
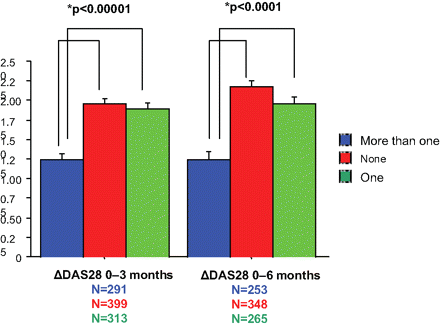
The mean (SD) improvement in DAS28 at 6 months for patients with no or one previous biological treatment was 2.2 (1.4) and 2.0 (1.4), respectively, while for patients for whom two or more biological agents had failed, the improvement was 1.3 (1.6) (figure 3). The difference between the first two groups and the third group was statistically significant (p<0.0001 at 3 and 6 months). At 6 months, the percentage of patients who achieved EULAR good/moderate response was 23.1%/50.6%, 20.5%/46.6% and 20.2%/29.8% for patients with no, one or more than one previous biological agents, respectively.
Mean DAS28 improvement (bars: SEM) for patients who failed none, one or more than one biologic DMARD. The mean reductions in DAS28 during the first 3 and 6 months were significantly greater for patients having failed at most one biologic compared to those who had two or more prior biologics (p<0.0001).
Predictors of response
Possible predictors of EULAR good response at 6 months were identified by univariate logistic regression analysis adjusted for age and sex (table 3). In the subsequent multivariate analysis previous use of ≤1 versus >1 biological DMARDs, lower baseline DAS28 level and anti-CCP positivity were significant predictors of EULAR good response, while a smaller number of previous synthetic DMARDs was borderline significant (table 3). Since only a fraction of patients had available anti-CCP status, we performed a second multivariate analysis without anti-CCP and obtained the same results, but with somewhat stronger levels of significance (data not shown).
EULAR good response: univariate and multivariate logistic regression analyses
Discussion
In this large observational cohort study, the majority of patients treated with RTX had longstanding RA and several previous synthetic DMARDs and/or at least one anti-TNF had failed as in several randomised clinical trials with RTX.1 2 In addition, there was a significant off-label usage of RTX in anti-TNF naive patients.21 22 The heterogeneous population with data from 10 countries allowed us to evaluate the real-life effectiveness of RTX and stratification according to number of previous biological agents and seropositivity led to relevant conclusions.
Clinical effectiveness of RTX during the first 6 months after a treatment course was confirmed in this observational study. Previous studies have indicated that RF seropositivity is a predictor of response to treatment, which is expected since patients with RA most likely to respond to treatment with RTX are those in whom B cells play a more important role in the pathogenesis of their disease. Such patients could potentially be identified by analyses of serum autoantibodies, including RF and anti-CCP.1 23 24 The study confirms this observation, as significantly better results were seen after 6 months for RF-positive patients than for RF-negative patients, but also for anti-CCP-positive versus negative individuals and double-positive versus double-negative subjects. However, seronegative patients also responded well to RTX. The difference between seropositive and seronegative patients at 6 months was not as strong as at 3 months. Moreover, DAS28 reduction from 3 to 6 months did not differ between the two groups. One possible explanation is that seronegative patients respond more slowly than patients who are seropositive. While initial reports suggested a lack of effectiveness of RTX in seronegative patients,4 25 more recent studies have suggested that RTX can be effective for such patients as well.1 2 These results could be explained by the observation that B cells play an important role in the pathogenesis of RA as autoantibody producers and also as antigen-presenting cells and proinflammatory cytokine-releasing cells. It is also theoretically possible that seronegative responders produce autoantibodies not as yet known. There is, however, a risk of underlying bias: only seronegative patients with a satisfactory response continue to receive RTX for 6 months, and therefore the patients for whom we have 6 months' data could be selected from the initial population. To recommend use of RTX in seronegative patients further support from large observational or clinical studies is needed.
Anti-CCP was found in the univariate and multivariate analysis to be a significant independent prognostic marker of EULAR good response after 6 months, with an OR of 2.86 (95% CI 1.43 to 5.71). RF positivity was shown to be a significant predictor of EULAR good response in the univariate analyses, but did not remain significant in the multivariate model, even though the patients numbers with information about RF was much higher than the number with information about anti-CCP. These observations may indicate that anti-CCP positivity is a stronger predictor of response to RTX than RF seropositivity. Longer follow-up, larger patient numbers and prospective data collection will allow us to reach more robust conclusions about seropositivity and response to RTX in the future.
In this study a large proportion of patients (36.6%) received RTX before having received an anti-TNF. RTX has more recently been tested in randomised controlled clinical trials of inadequate responders to synthetic DMARDs.21 22 Future examination of the specific reasons for use of RTX as first biological agent might be of value. Patients naïve to anti-TNF agents and other biological agents, as well as patients for whom only one previous biological agent has failed seemed to respond significantly better to treatment than patients for whom two or more biological agents had failed. This observation suggests that earlier initiation of RTX might lead to better results. Alternatively, lack of response to two or more anti-TNF agents might suggest resistance to treatment. The latter suggestion was supported by the lack of association between delay to RTX treatment (baseline disease duration) and response.
Observational studies have indicated that RTX might be a better alternative than switching between TNF antagonists after a failure of anti-TNF treatment.26 27 We had no data available to examine this question in this analysis, but this research question may be addressed later in the CERRERA database.
Another interesting result was a slight decrease in effectiveness of treatment between 3 and 6 months for patients not being co-treated with DMARDs. Thus, concomitant DMARDs seem to maintain the therapeutic effect of RTX. In future analyses the effect of different DMARDs in combination with RTX will be examined.
This study started with 2019 patients. However, the number of patients analysed at 3 and 6 months was lower (figure 1). To exclude an important bias, we compared the characteristics of patients missing at these time points with those used for the analysis, which was particularly relevant for evaluation of the responses of seronegative patients with RA. As shown in figure 2B, no difference in the number of anti-CCP-positive and anti-CCP-negative patients with available data at 3 and 6 months could be detected (61.6% vs 68.1% and 46.7% vs 48.6%, respectively). Contrarily, significantly more RF-negative patients than RF-positive patients had available DAS28 at 3 (61.3% vs 52.3%) and 6 months (56.5% vs 44.6%) (figure 2A). The last observation could support our finding that seronegative patients can also respond well to RTX but more slowly than those who are seropositive. On the other hand, one could suggest that the missing data of a large amount of seropositive patients, especially at 6 months, might be related to a very good response to treatment, thus introducing a selection bias. The absence of difference in the anti-CCP subgroup allowed us to reach more robust conclusions on anti-CCP positivity as a predictor of response to RTX.
This study has some limitations, such as the significant heterogeneity between countries for baseline characteristics, differences in laboratory methods for determining RF and anti-CCP antibodies and the difficulty of splitting the countries into groups in order to include them as a covariate in the multivariate analysis. Another weakness is that the study was observational and uncontrolled. Perhaps some of the improvements can be explained by regression to the mean or the placebo effect. Also, we are using observed data and we cannot report whether patients who dropped out before follow-up visits or had missing data also had worse results. The quite high number of missing data is of concern, but it is something we expected, as it is a large observational cohort. On the other hand, the large number of patients, the fact that there are 10 participating countries with different treatment protocols, the ‘off-label’ treatment of anti-TNF naïve patients with RTX, the possibility of examining the combination of RTX with other DMARDs apart from MTX, and the fact that all data came from ‘real-life’ patients are significant strengths of this study.
In conclusion in this large observational cohort, RF-positive and anti-CCP-positive patients achieved significantly greater reductions in DAS28 at 6 months than seronegative patients. Anti-CCP positivity was an independent predictor of EULAR good response. Effectiveness results were best when RTX was used as the first biological agent or in patients for whom at most one anti-TNF had failed. Concomitant DMARDs may prolong the initial effectiveness, leading to longer relapse-free disease.
References
Supplementary materials
Web Only Data
Files in this Data Supplement:
Footnotes
-
Ethics approval This study was conducted with the approval of the registry of each country.
-
Provenance and peer review Not commissioned; externally peer reviewed.