Article Text
Abstract
Objective Early diagnosis of spondyloarthritis (SpA) is sometimes difficult owing to the lack of reliable diagnostic criteria. The objective of this study was to determine the diagnostic accuracy of detecting enthesitis by power Doppler ultrasonography (PDUS) in patients with suspected SpA.
Methods A prospective single-centre cohort study was performed in patients with symptoms suggestive of SpA (inflammatory back pain, arthritis, enthesitis or dactylitis, HLAB27+ uveitis) who underwent clinical examination, pelvic x-ray, MRI of lumbar spine/sacroiliac joints, HLA-B typing and other tests judged useful for diagnosis. Blinded PDUS examination of seven sites of enthesitis was performed at baseline. The gold standard was the diagnosis made by the referring rheumatologist according to the development of symptoms and findings, blinded to PDUS results, during routine follow-up for up to 2 years.
Results Between November 2002 and October 2004, 118 patients were included in the study. After 2 years a definite diagnosis was retained for 99 patients (51 SpA and 48 non-SpA). PDUS detection of at least one vascularised enthesis provided good predictive value for diagnosing SpA (sensitivity 76.5%; specificity 81.3%; positive likelihood ratio 4.1; OR 14.1; p<0.0001). Vascularised enthesitis detected by PDUS and Amor's criteria were the only independent contributors to a diagnosis of SpA in multivariate logistic regression (c-index=0.87). Alternatively, CART analysis resulted in a highly sensitive and specific diagnostic tree by combining PDUS with Amor's criteria.
Conclusions PDUS appears to be a valuable first-line diagnostic tool to confirm a diagnosis of SpA.
Statistics from Altmetric.com
Spondyloarthritis (SpA) is one of the most frequent varieties of inflammatory rheumatic disorders with an estimated prevalence of 0.1–1% in Caucasians1 2 and a strong genetic predisposition dominated by its association with HLA-B27.3 The prototypical symptom of SpA is inflammatory back pain (IBP). Other manifestations are peripheral arthritis, enthesitis, acute anterior uveitis (AAU), psoriasis and inflammatory bowel disease (IBD).4
Peripheral enthesitis, an important feature that can be observed in all forms of SpA, usually manifests as isolated pain or tenderness at physical examination, but is sometimes only detected by imaging techniques.5,–,7 One major hurdle faced by clinicians is their inability to establish an early diagnosis because of the poor specificity of symptoms of SpA.7 The detection of inflammation markers is inconsistent.8 The presence of HLA-B27 is more sensitive but has incomplete positive predictive value because of its relatively high prevalence in the population. Finally, advanced radiographic sacroiliitis, which is a hallmark of ankylosing spondylitis (AS), takes years to develop.9 10
Two classification criteria sets have been developed to embrace the entire SpA spectrum: Amor's criteria and the European Spondylarthropathy Study Group (ESSG) criteria.11 12 Both sets have limitations, especially in early disease. The specificity of Amor's criteria is quite high but the sensitivity is low, while the ESSG criteria have higher sensitivity but low specificity.13 14 Thus, neither set is entirely satisfactory for an early diagnosis of SpA.13 14 The ability of MRI to detect sacroiliitis and spondylitis in patients without radiographic signs is well established,15 resulting in its inclusion in the two most recent classification criteria developed for axial SpA—the Berlin criteria16 and the Assessment of SpondyloArthritis international Society (ASAS) criteria.17 18 The specificity of MRI is high but the sensitivity falls when applied to early axial SpA (around 65%)18 or to the entire group of SpA (axial and peripheral). To overcome this the ASAS has recently proposed a set of criteria for patients with predominantly peripheral manifestations.19
Ultrasonography (US) has a greater sensitivity than clinical examination and other imaging techniques for the detection of peripheral enthesitis.20 21 In a cross-sectional study using US in B mode combined with power Doppler (PDUS), we observed a higher frequency of peripheral enthesitis in patients with SpA than in controls, irrespective of SpA subtype. The landmark feature of enthesitis was an abnormal vascularisation of entheses which was exclusively detected in SpA.22 This observation has been confirmed by others.23 24 In the present study we estimated the diagnostic accuracy of PDUS in patients with suspected SpA. A further objective was to establish the best diagnostic strategy by combining PDUS of entheses, MRI of spinal/sacroiliac joints and existing classification criteria.
Methods
Patients
Outpatients consecutively referred to our rheumatology department between November 2002 and October 2004 for symptoms suggestive of SpA were considered eligible for enrolment in this prospective study. The protocol was approved by the institutional ethics committee and all patients gave written informed consent to participate. Inclusion symptoms were: (1) IBP; (2) arthritis or inflammatory arthralgia; (3) enthesitis or dactylitis; or (4) AAU in a HLA-B27+ individual. For IBP, arthritis, arthralgia and enthesitis, symptom duration >3 months and age <50 years were required. In patients with several inclusion criteria, the most prominent symptom was considered as the primary one (definitions of inclusion criteria are shown in the online supplement). Patients were excluded if they were aged <18 years or if a definite diagnosis of AS or other well-defined disease accounting for the presenting manifestation(s) was made during the eligibility visit. Patients were followed up for 2 years by their referring rheumatologist, who was asked every 6 months whether a definite diagnosis had been ascertained.
Assessment
Clinical and laboratory evaluations
At baseline all patients were submitted to a standardised clinical examination by a qualified rheumatologist who was blind to the diagnosis suspected by the referring rheumatologist. Past medical history and symptoms present at baseline (see online supplement) were collected. Patients with AAU referred by an ophthalmologist were examined according to the rheumatological symptoms recorded at baseline. In the absence of any rheumatological manifestation, a detailed physical examination (axial and peripheral) was performed. These patients were subsequently referred to a rheumatologist for follow-up. Laboratory evaluations helpful for diagnosis (ie, HLA-B typing, autoantibodies, acute phase reactants and biochemistry) were performed in all patients.
Radiological and MRI evaluations
A pelvic x-ray not older than 6 months was obtained for all patients. Sacroiliitis was scored according to the modified New York criteria.25 In cases of doubtful radiographic sacroiliitis and/or in cases of buttock pain persisting for >6 months, a pelvic CT scan was performed. The detection of advanced sacroiliac structural changes (ie, erosions, ossification) was considered as definite sacroiliitis. Other x-rays of the spine or joints were performed if needed for diagnosis.
Gadolinium MRI (see online supplement for sequences) of sacroiliac joints and lumbar spine (if symptomatic) was performed in patients with past or present IBP. The results were categorised into presence or absence of active inflammatory lesions (ie, intense bone oedema, detection of synovitis or enthesitis after gadolinium enhancement) and/or chronic lesions (ie, erosions) consistent with SpA. All CT scans, x-rays and MRI were assessed by an expert radiologist blinded to clinical symptoms.
PDUS evaluation
At baseline, all patients underwent PDUS examination of peripheral entheses using an Esaote Technos MPX (Esaote, Genoa, Italy) by an independent examiner (MAD'A) who was blinded to the subject's identity and symptoms. The following entheseal insertions were examined bilaterally in both planes: plantar fascia, Achilles tendon, patellar ligament on the patella apex, quadriceps femoris, gluteus medius tendon, common extensor and common flexor tendons on the lateral and medial epicondyle of the elbow. The sonographer looked for morphological and structural abnormalities in B mode and vascularisation with power Doppler at bony insertions. The PDUS technical parameters, definition and detection of elementary lesions and PDUS global score are shown in the online supplement. Three criteria were evaluated for diagnostic performance: the detection of any vascularised enthesis; the number of abnormal entheses; and the global PDUS score. Figure 1A,B shows the minimal and clear aspects of PDUS abnormalities of entheses in study patients.
(A) Power Doppler ultrasonography pattern of minimal enthesitis of the epicondyle using a longitudinal scan: normal aspect in B mode and minimal vascularisation (V) by power Doppler at the cortical junction. (B) Power Doppler ultrasonography pattern of established Achilles tendon enthesitis using a longitudinal scan: abnormalities in B mode are hypoechogenicity of enthesis insertion associated with small enthesophyte (En), erosions of cortical bone (Er) and abnormalities by power Doppler:V= vascularisation at the cortical junction.
Diagnostic decision
The diagnosis retained by the referring rheumatologist according to his routine practice after a follow-up period up to 2 years was used as the gold standard (ie, dependent variable) against which the performance of PDUS findings, classification criteria and clinical manifestations retrieved at inclusion (ie, independent variables) were assessed. Referring rheumatologists were informed of the results of laboratory, radiographic and MRI examinations but not of PDUS.
Statistical analysis
Comparisons between groups for continuous and ordinal data were performed by t test or Kruskall–Wallis test depending on sample size, and by a χ² test (Fisher exact test when appropriate) for categorical data. The number of entheses involved by PDUS at each site (ie, none, one side, two sides) was compared between groups using the Cochran–Armitage trend test. The Wilcoxon rank test was used for evaluating the association between PDUS and MRI findings. Tests were two-tailed with 5% type I error. Sensitivity, specificity, positive and negative likelihood ratios (LR+, LR−) of PDUS, SpA criteria (ESSG, Amor's, Berlin and ASAS) and other diagnostic tests were estimated (with 95% CI). Amor's criteria were evaluated either as a binary variable (yes if score ≥6, otherwise no) or as elementary items composing the set.
To determine the independent contribution of each parameter to the diagnosis of SpA a multivariate model was developed. First, the strength of the association between PDUS findings, clinical parameters, classification criteria, other imaging techniques and the primary outcome (ie, diagnosis of SpA) was estimated by univariate logistic regression analysis. Variables associated with the outcome measure (p≤0.20) were introduced in a logistic regression model following a stepwise selection until no variables met the criteria for entry (p<0.05) or removal (p≥0.05), observed on LR test. The discriminant power of the final score calculated from the logistic equation was evaluated by the c-index (equivalent to the area under the receiver operating characteristic (ROC) curve), while calibration of the model was assessed by the Hosmer–Lemeshow test. An alternative model relying on recursive partition methodology (CART) was also developed (see online supplement for explanation of CART). Internal validation and determination of CI were performed by resampling procedures. The effect of missing data was checked by sensitivity analysis. Statistical analysis was performed with SAS Version 9.1 (SAS Institute, Cary, North Carolina, USA) and R Version 2.6 (http://www.r-project.org/).
Results
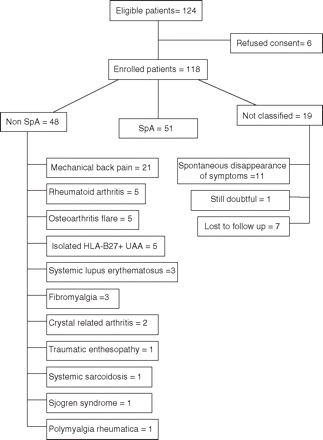
One hundred and eighteen of 124 eligible patients consented to participate to the study. Table 1 shows the baseline demographic characteristics, primary inclusion criteria, past clinical history, all clinical and imaging characteristics and HLA-B27+ of the enrolled patients. Pelvic MRI was performed for 83 patients, of whom 75 also underwent lumbar MRI. At the end of the follow-up period 99 patients were classified by their referring rheumatologist (51 (43%) SpA, 48 (41%) non-SpA) and 19 (16%) remained unclassified (figure 2). No difference was observed in the distribution of primary inclusion criteria between SpA and non-SpA patients. However, isolated inflammatory buttock pain, the efficacy of non-steroidal anti-inflammatory drugs in treating IBP, a family history of SpA, HLA-B27 positivity and positive MRI findings (pelvic or lumbar) were more prevalent in patients classified as SpA than in those classified as non-SpA (p<0.05 in all comparisons, table 1).
Patient flow chart. SpA, spondyloarthritis.
Demographic, clinical, laboratory and radiological characteristics of patients and classification criteria at baseline
At baseline some patients fulfilled established classification criteria for SpA (eg, Amor's, ESSG, Berlin or ASAS axial), the last two being applied only to patients with IBP who underwent a pelvic MRI, and ASAS peripheral which was applied only to patients with clinically confirmed peripheral manifestations (table 1). There was a statistically significant difference between SpA and non-SpA patients for all the above criteria (p<0.0001, table 1).
PDUS findings
Eighty-eight of 118 patients (75%) who underwent PDUS had at least one abnormal enthesis. Of these, 56 (64%) had at least one vascularised enthesis (39 (76%) in SpA, 9 (19%) in non-SpA and 8 (42%) in unclassified patients).
The number of abnormal entheses per patient detected by PDUS was significantly greater in patients with SpA than in non-SpA patients (p<0.01), as well as the number of vascularised entheses (p<0.0001) and the prevalence of at least one abnormal or of one vascularised enthesis (p=0.003 and p<0.0001, respectively). Overall, more severe grading in the PDUS global score was observed in SpA than non-SpA (p<0.0001). Table 2 shows the detailed distribution of PDUS findings and PDUS scores. Achilles and lateral epicondyle tendons were more frequently involved in patients with SpA than in non-SpA patients (Cochran–Armitage trend test, p=0.01 and p=0.03, respectively).
Power Doppler ultrasonography (PDUS) findings at baseline
The global PDUS score was statistically significantly associated with the presence of MRI inflammatory signs (Wilcoxon test, p=0.027). Inflammation at MRI was also significantly associated with the number of vascularised entheses (p=0.009) but not with the total number of entheses involved at PDUS (p=0.112).
In patients with peripheral symptoms at baseline or previously, low agreement was observed between clinical symptomatic findings and their detection by PDUS. This applied to the number of entheses (AUC-ROC curve=0.65) or the presence of at least one positive enthesis by PDUS (κ=0.24). A relatively better agreement was observed between clinically detected findings and the number of vascularised entheses (AUC-ROC curve=0.78) or the presence of at least one vascularised enthesis (κ=0.52). It was noteworthy that, in the subset of patients fulfilling ASAS peripheral criteria, poor agreement was observed between clinical and PDUS findings for both the total number of vascularised entheses (AUC-ROC curve=0.45) or the detection of at least one vascularised enthesis (κ=0.02).
Diagnostic performance of PDUS inflammatory enthesitis and classification criteria at baseline
The diagnostic accuracy of the most relevant PDUS findings with regard to the final diagnosis is shown in table 3.
Performance of power Doppler ultrasonography (PDUS) findings, classification criteria, other manifestations at baseline for predicting the final diagnosis of SpA in classified patients
A weak association was observed between the number of abnormal entheses by PDUS, the global PDUS score (OR 1.2 (95% CI 1 to 1.3), p<0.01, c-index= 0.65; and OR 1.1 (95% CI 1 to 1.2), p=0.0005, c-index=0.72, respectively) and the diagnosis of SpA. Conversely, a strong association was found with the number of vascularised entheses (OR 4.4 (95% CI 2.4 to 8.7), p<0.0001, c-index=0.80) and the detection of at least one vascularised enthesis (sensitivity 76.5%; specificity 81.2%; LR+ 4.1; LR− 0.3; OR 14.1; p<0.0001, c-index=0.79; table 3).
We also evaluated the diagnostic performance of each established classification criteria system and of the single manifestations composing those sets. Among all the criteria applicable to the entire population (ie, ESSG and Amor), the best diagnostic performance was achieved by Amor's criteria (sensitivity 60.2%; specificity 87.5%; LR+ 4.8; LR− 0.45; OR 10.8; p<0.0001, c-index=0.74; table 3). The diagnostic performance of the new sets of criteria (Berlin, ASAS axial and peripheral) applied to the subgroups of interest (ie, axial and peripheral presentation) is also shown in table 3. We also determined whether one vascularised enthesis could be a valuable candidate criterion for the ASAS peripheral criteria set. By adding this PDUS criterion (ie, ASAS positive and PDUS positive), we increased the specificity of those criteria from 86.7% to 100% but sensitivity was reduced from 50.0% to 41.7%. On the other hand, using PDUS as an alternative diagnostic criterion (ie, ASAS positive or PDUS positive) increased the sensitivity (83.3%) without losing much specificity (80%). For further details see table A in the online supplement.
Multivariate analysis and classification tree
In the whole cohort, two independent contributors predicted the final diagnosis of SpA: (1) at least one vascularised enthesis (OR 11.89 (95% CI 4.3 to 37.2); p<0.0001); and (2) Amor's criteria (OR 8.8 (95% CI 2.9 to 31); p<0.001), with a c-index of 0.87. Increasing the number of vascularised entheses (ie, 2, 3 or more) did not improve the prediction (data not shown). Other characteristics, as well as other classification criteria (ie, ASAS and Berlin when applied only to the population of interest—that is, patients with axial and peripheral symptoms), even if statistically associated with the diagnosis of SpA, were not independent contributors (data not shown).
Moreover, by stratifying patients with and without peripheral symptoms, the results of multivariate analysis did not change. Independent contributors still remained at least one vascularised enthesis and Amor's criteria (OR 11.2 (95% CI 3.9 to 32.4), p<0.0001; and OR 8.4 (95% CI 2.5 to 28.4), p<0.0001, respectively).
CART analysis resulted in two main diagnostic algorithms (figure 3A,B). In the first algorithm the detection of at least one vascularised enthesis by PDUS would lead to a positive diagnosis of 70% of patients as SpA. If no vascularised enthesis was detected, the positivity of Amor's criteria predicted 54% of those patients as having SpA. Using this tree yielded a sensitivity of 90%, specificity of 77% and LR+ of 3.94 (figure 3A). An alternative way would be to use Amor's criteria first. In this case the detection of at least one vascularised enthesis in patients not fulfilling Amor's criteria would predict 56% of those patients as having SpA and the absence of any vascularised enthesis would predict 76% of them as non-SpA. Using this tree yielded a sensitivity of 79%, specificity of 82% and LR+ of 4.45 (figure 3B). Simulations to assess the influence of varying prevalence of SpA did not modify these conclusions (data not shown).
Decision tree for patients with suspected spondyloarthritis (SpA). (A) All suspected patients. (B) Patients who do not fulfil Amor's criteria (score <6). PDUS, power Doppler ultrasonography. For additional explanation see Results section.
Discussion
To our knowledge, this is the first prospective cohort constructed to evaluate the accuracy of a diagnostic technique such as PDUS in patients with suspected SpA. Diagnosing SpA is difficult owing to the absence of a gold standard, even if several tests have been available to aid the SpA diagnosis. However, some of them have insufficient sensitivity in some situations (x-rays, MRI) or incomplete specificity (HLA-B27 typing).26 Thus, the high sensitivity and specificity of detecting enthesitis by PDUS in established and early SpA led us to consider it as a candidate diagnostic tool.22 24 27
Several important findings resulted from this study. First, we showed that the detection of enthesitis by PDUS had a high predictive value for a diagnosis of SpA in patients with suspected SpA. In this cohort the prevalence of peripheral enthesitis was independent of the clinical phenotype (axial or peripheral), consistent with observations in established SpA.22 In agreement with our previous observation and recent Spanish studies, we confirmed that the number of entheses involved and the PDUS global score could distinguish SpA from non-SpA with good sensitivity and specificity.22 24 27 Strikingly, we also confirmed that vascularisation of the enthesis insertion by PDUS is a landmark feature for SpA, even in suspected cases. Both logistic regression and CART analysis underlined the high diagnostic value achieved by this feature. One vascularised enthesis was sufficient to predict a diagnosis of SpA, independent of the localisation and the frequency of involvement. We considered the diagnosis by the rheumatologist as a reference, given the lack of gold standard.28 The large number of rheumatologists (n=17) and the fact that they classified patients without any formal consensus rules could be seen as a limitation. Presumably, however, they used the widely validated classification criteria as well as pelvic MRI results to help diagnose SpA. This could account for the high specificity of Amor's criteria with regard to the final diagnosis, as well as that of the ASAS criteria, which combine both clinical and imaging findings. In our cohort we observed a significant correlation between MRI and positive PDUS findings, which supports the validity of PDUS for detecting SpA.
It is therefore all the more remarkable that the best independent imaging variable strongly predicting SpA was PDUS detection of enthesitis at baseline, which was ascertained blindly. In this study, which was performed in a tertiary rheumatology care centre, the median duration of symptoms at entry was quite long. This can be interpreted as a potential limitation for the validity of the use of PDUS in early SpA. On the other hand, it could account for the large proportion of patients who were classified by their referring rheumatologist within 2 years. It is also worth mentioning that, of 20 patients referred for HLA-B27+ AAU, the 7 for whom a diagnosis of SpA was not retained were positive by PDUS (5 non-SpA, 2 lost to follow-up) and would have been classified as SpA, despite a lack of obvious rheumatological symptoms, using the decision tree resulting from CART analysis. This is in line with a study showing enthesitis by PDUS in isolated patients with HLA-B27+ AAU, similar to that seen in full-blown SpA.29 This interpretation is also consistent with a genetic study which showed that AAU in the context of SpA is dependent on the same factors as other manifestations of SpA.10 It is also supported by the 5-year follow-up of one study patient who was diagnosed as having AS after a new episode of AAU based on typical radiographic sacroiliitis and lumbar syndesmophytes. This patient had a positive pelvic MRI at baseline and the only rheumatological symptom since inclusion was mechanical back pain.
PDUS detection of enthesitis is a delicate technique, and the choice of equipment and the sensitivity of Doppler is of primary importance for evaluating vascularisation in such small sites and should be taken into account for the generalisability of results. However, it can be performed reliably by sonographers with varying levels of experience following dedicated training.30
Our CART analysis suggested two alternative diagnostic procedures in patients with symptoms suggestive of SpA, the order of which may depend on the setting. The priority use of PDUS or Amor's criteria could therefore be decided according to the feasibility of PDUS examination, on the one hand, and the level of clinical evidence allowing a diagnosis using classification criteria on the other. In cases where sufficient clinical and paraclinical criteria are absent and the suspicion still persists, we suggest that PDUS should be performed.
References
Supplementary materials
Web Only Data
Files in this Data Supplement:
Footnotes
-
Funding This study was supported by a grant from the French Society of Rheumatology (SFR) in 2004 (15 000 Euros) used for data acquisition, creation of a database and analysis.
-
Competing interests None.
-
Ethics approval This study was conducted with the approval of the Ambroise Paré Hospital, Boulogne-Billancourt France.
-
Provenance and peer review Not commissioned; externally peer reviewed.