Article Text
Abstract
Objective To define the specificity and extent of duplex sonography (DS) findings suggestive of vessel wall inflammation in patients with giant cell arteritis (GCA).
Methods Patients admitted between December 2006 and April 2009 to the University Hospital Basel with a suspicion of GCA were eligible for the study. DS of 2×11 arterial regions was performed in all study participants, and American College of Rheumatology criteria were applied to classify patients into GCA or non-GCA groups.
Results GCA was diagnosed in 38 of the 72 participants (53%). A DS pattern suggestive of vessel wall inflammation was not observed in any of the patients in the non-GCA group but, in 21 of the 38 patients with GCA (55%), DS signs suggestive of vessel wall inflammation of ≥1 vessel region were detected. In 12 of the 38 patients with GCA (32%), DS signs of large vessel vasculitis (LVV) were found in ≥1 vessel region(s) of both upper and lower limb vessels. Follow-up DS was performed 6 months after the baseline examination in 9 of the 12 patients with LVV and showed the persistence of most findings despite normalised signs of systemic inflammation.
Conclusion DS detects changes in the vessel wall that appear to be specific for GCA; they can be present in upper and lower limb arteries of patients with GCA. Surprisingly, DS-detectable LVV and signs of systemic inflammation are largely dissociated.
Statistics from Altmetric.com
Introduction
In patients with suspected giant cell arteritis (GCA), a hypoechoic halo detected by duplex sonography (DS) around temporal arteries has been suggested to identify vessel wall inflammation.1 Recently, in a prospective evaluation of the arteries of the upper extremities, hypoechoic lesions suggestive of vasculitis were observed in around 30% of patients with GCA.2 Positron emission tomography (PET) studies in patients with GCA also showed metabolic activity suggestive of inflammation in the wall of extracranial large arteries, and several case reports/series—including reports of histopathological examination of lower limb arteries—have substantiated the notion that vessel wall inflammation in GCA can affect large vessels throughout the body.3,–,5
Suspected inflammation of the temporal artery can be directly assessed histologically. By contrast, histological verification of large vessel vasculitis (LVV) suspected in DS examination is rarely possible, and uncertainty about the specificity of DS in this setting remains.
By assessing a non-selected cohort of patients admitted with suspicion of having GCA, we aimed to define: (1) how DS findings suggestive of large vessel inflammation relate to the diagnosis of GCA based on American College of Rheumatology (ACR) criteria and (2) the extent of peripheral ‘vasculitic’ large vessel involvement as captured by DS.
Methods
Patients
Patients admitted to the University Hospital Basel between December 2006 and April 2009 with suspicion of having GCA were eligible for the study. ACR criteria were used to establish the diagnosis of GCA.6 One patient with LVV according to PET criteria, an erythrocyte sedimentation rate (ESR) >50 mm/h and aged >50 years was included as waiver.7
Duplex sonography
An iU22 duplex device (Philips, Best, The Netherlands) with a linear 3–9 and 5–17 MHz transducer was used. The following arterial segments were screened bilaterally using B-mode and colour Doppler: the common carotid artery from its origin to the bifurcation, the extracranial part of the internal and external carotid artery, the vertebral artery (entire extracranial length, segments V0–V3), the subclavian and axillary arteries, the common superficial temporal artery with its parietal and frontal branch, the common femoral artery, the deep femoral artery, the proximal and mid part of the superficial femoral artery and the popliteal artery. Spectral traces were obtained routinely from the carotid, vertebral, subclavian and femoral arteries in other segments only when indicated by colour Doppler analysis. The temporal artery was routinely scanned in longitudinal and cross-sectional views; the other segments were examined in the longitudinal view, with cross-sections only if pathologies were suspected.
The DS findings in each segment were categorised as ‘normal’, ‘arteriosclerosis’ or ‘vasculitis’. Irregularly delineated, non-homogenous, eccentric or calcified wall alterations were defined as ‘arteriosclerosis’. ‘Vasculitis’ was defined as follows: for the temporal artery as suggested by Schmidt et al1 for larger arteries, circumferential homogenous hypoechoic wall thickening (with or without stenosis), well-delineated towards the luminal side and absence of arteriosclerotic lesions. In lower limb arteries an echolucent stripe within the wall thickening was considered as an additional sign of vasculitis.5 Findings not clearly classifiable as ‘vasculitis’ were initially recorded as ‘suspicious for vasculitis’ and reclassified as ‘vasculitis’ if at least one other segment was defined as ‘vasculitis’ and as ‘arteriosclerosis’ in the remaining cases. Both experienced angiologists performing DS were blinded to the clinical classification of study participants; DS did not influence the classification. Figure 1 shows representative ultrasound images of extratemporal vessels classified as ‘normal’, ‘arteriosclerosis’, ‘vasculitis’ and ‘suspicious for vasculitis’.
Representative ultrasound images. Arterial segment ultrasound images classified as (A) ‘normal’ showing a thin homogenous intima/media layer; (B) ‘arteriosclerosis’ with eccentric irregular plaques and acoustic shadowing; (C) ‘vasculitis’ with homogenous hypoechoic wall broadening; and (D) ‘suspicious for vasculitis’. Arrows identify the vessel wall.
Statistical analysis
Categorical variables were compared between patients with and without GCA and between subgroups of patients with GCA using the Pearson χ2 test; continuous variables were analysed using the Mann–Whitney U test. Linear regression analysis was performed to assess the influence of disease and age on the number of atherosclerotic vessel regions.
Results
Patient characteristics
Seventy-two patients were included in the study, 38 of whom (53%, 15 men) fulfilled the ACR criteria for GCA. The remaining 34 patients (47%, 12 men) were diagnosed with polymyalgia rheumatica (n=12), headache (n=3), antineutrophil cytoplasmic antibody-associated vasculitis (n=3), undifferentiated connective tissue disease (n=2), polyarthritis (n=2), weight loss of unknown origin (n=2), aortic aneurysm (n=2), cerebrovascular insult (n=2) and one patient each with arteritic ischaemic optic neuropathy not related to GCA, fever of unknown origin and sarcoidosis and rotator cuff degeneration. In two patients no diagnosis was made. The median age at study entry was 73 years (range 57–94) in the patients with GCA and 64 years (range 27–89) in the non-GCA group. In 37/38 patients (97%) with GCA, a temporal artery biopsy was performed which was diagnostic in 95%. In 19/34 (56%) patients in the non-GCA group a temporal artery biopsy was performed but did not yield any positive results. In patients with GCA, median C-reactive protein (CRP) levels were 65 mg/l (range 4–256) and median ESR was 70 mm/h (range 6–120). Patients were followed up for 5–34 months (mean 18). No alternative diagnoses became apparent during this time.
DS was performed a median of 1 day before initiation of steroid treatment (range 7 days before initiation to 10 days after initiation of treatment). In 9 of the 12 patients with extratemporal vasculitis as defined by DS, a follow-up DS study of the extratemporal arteries was performed 6 months after study entry.
Robustness of the DS classification criteria
A total of 1522 arterial segments (96% of intended) were analysed. In 42/1522 assessments (3%) the DS findings were classified as ‘suspicion of vasculitis’. However, clearcut DS findings of ‘vasculitis’ or ‘arteriosclerosis’ (one patient) in other arterial segments permitted unambiguous final classification.
Relation of DS findings to ACR-based clinical diagnosis
A DS pattern classified as ‘vasculitis’ was not seen in any of the 34 participants in the non-GCA group. In contrast, in 21/38 patients with ACR-defined GCA (55%), ≥1 vascular segment was classified as ‘vasculitis’ according to DS findings. The sensitivity of DS for a diagnosis of GCA was 55%, specificity 100%, positive predictive value 100% and negative predictive value 67%.
In 9 of the 21 patients with GCA and DS findings classified as ‘vasculitis’, only the temporal arteries were involved. In the remaining 12 patients at least one extratemporal site was classified as ‘vasculitis’ by DS, indicative of LVV. Upper and lower extremity arteries were involved in 60% and 55%, respectively. In 5 of the 12 patients, stenoses >50% of the vessel lumen were observed in 1–2 vessel regions. The distribution of ‘vasculitis’ classified by DS in these 12 patients is summarised in table 1.
Distribution of duplex sonographic (DS) findings in patients with indication of large vessel vasculitis (LVV)
In 58 of the 72 study participants (81%) at least one vascular segment was classified as ‘arteriosclerosis’. In patients fulfilling the ACR criteria for GCA, a median of 7 (range 0–22) vascular segments were classified as ‘arteriosclerosis’ compared with 4 (range 0–22) in the non-GCA group. After adjustment for age, the number of vascular segments classified as ‘arteriosclerosis’ did not differ between the GCA and non-GCA groups (p=0.81).
Relation of extratemporal DS findings to clinical ischaemia
Of the eight patients with ‘vasculitis’ of the lower limb arteries classified by DS, two presented with claudication. None of the patients with DS-classified ‘vasculitis’ of the upper limbs presented with signs or symptoms of arm ischaemia.
Clinical differences between patients with and without LVV
Weight loss was more prevalent among the 12 patients with DS-classified LVV than in the 26 patients with GCA without DS findings of extratemporal vasculitis (67% vs 5%, p<0.001). The two groups did not differ in age, sex, incidence of vision loss, jaw claudication, headache at presentation, ESR at presentation (82 mm/h vs 65 mm/h, p=0.38) and CRP level at presentation (59 mg/l vs 63 mg/l, p=0.59).
Follow-up DS findings in patients with LVV
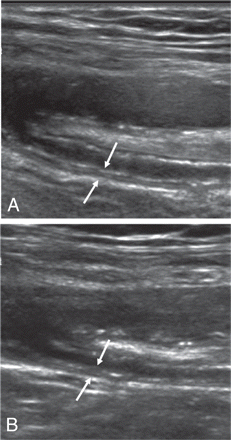
Nine of the 12 patients classified as LVV received a follow-up DS examination 6 months after inclusion in the study. Of the 84 vascular segments classified as ‘vasculitis’ at baseline, only 8 had a normal DS pattern whereas, in the remaining segments, a marginally enhanced echogenicity of the vessel wall persisted (figure 2). In one patient two vessel segments were newly classified as ‘vasculitis’ (table 1).
Follow-up ultrasound study of a segment of the deep femoral artery. (A) Baseline study classified as ‘vasculitis’ showing homogenous hypoechoic vessel wall broadening (arrow) and (B) follow-up study 6 months later and in clinical remission; compared with the baseline ultrasound study the vessel wall appears only slightly more echogenic (arrow).
Discussion
The key findings of this extensive and systematic DS study in a cohort of 72 non-selected patients with a clinical suspicion of GCA were that (1) predefined sonomorphological characteristics are highly specific for patients fulfilling the ACR criteria of GCA and (2) DS-defined ‘vasculitis’ is not restricted to upper limb arteries and can, in fact, cause clinically relevant lower limb ischaemia.
As a non-invasive (although time-consuming) tool (up to 75 min/patient) able to visualise all large peripheral arteries, DS has great potential in diagnosing, staging and following patients with large vessel inflammation. Defining how distinct DS patterns (ie, those suggestive of vasculitis) relate to ACR-defined criteria of GCA is therefore a high priority. In our study, combining simple DS criteria with extensive assessment of peripheral arteries permitted unambiguous classification of DS lesions with a high specificity for ACR-defined GCA. This indicates that the observed sonomorphological findings in patients with GCA may indeed reflect a GCA-associated pathology.
Our data support the notion that GCA is a systemic LVV and adds to our understanding of the clinical spectrum of the disease. One limitation of our study is the small sample size—particularly of patients with lower limb vasculitis—and the incomplete availability of follow-up data. These preliminary findings should, however, stimulate prospective studies assessing the clinical importance of DS morphological signs of ‘vasculitis’ in extratemporal arteries on follow-up examinations, and the relation of these extratemporal DS signs to the inflammatory process in the wall of temporal (and possibly other cranial) arteries.8,–,10
References
Footnotes
FK, MA, CH and TD contributed equally to this work
-
Funding TD is supported by the ‘Freiwillige Akademische Gesellschaft’ (FAG) of Basel and by an EULAR bursary. MS is supported by Oncosuisse (OCS-02266-08-2008). CH is supported by the Swiss National Science Foundation (PP00B-114850).
-
Competing interests None.
-
Ethics approval This study was conducted with the approval of the Basel ethics board.
-
Provenance and peer review Not commissioned; externally peer reviewed.