Article Text
Abstract
Objective To evaluate golimumab's effect on MRI-detected spinal inflammation in ankylosing spondylitis (AS).
Methods Patients were randomly assigned to subcutaneous injections of placebo (n=78), golimumab 50 mg (n=138), or golimumab 100 mg (n=140) every 4 weeks. An MRI substudy comprising 98 patients (placebo n=23, 50 mg n=37, 100 mg n=38) at eligible MRI substudy sites had serial spine MRI scans (sagittal plane, 1.5T scanners, T1 and short tau inversion recovery sequences) at baseline and weeks 14 and 104. Two blinded (treatment, image order) readers independently evaluated MRI spinal inflammation using AS spine MRI-activity (ASspiMRI-a) scores; reader scores were averaged. Changes from baseline to weeks 14 and 104 were compared among treatment groups using analysis of variance on van der Waerden normal scores both with (post-hoc) and without (prespecified) adjustment for baseline ASspiMRI-a scores.
Results Median baseline ASspiMRI-a scores were lower in the 100 mg (3.5) than placebo (6.8) and 50 mg (7.8) groups. Median decreases in activity scores from baseline to week 14 were −0.5 for placebo, −3.5 for 50 mg (p=0.047 vs placebo), and −1.5 for 100 mg (p=0.14 vs placebo). After adjusting for baseline ASspiMRI-a score imbalance, significant improvements were observed with both 50 mg (p=0.011) and 100 mg (p=0.002) versus placebo. ASspiMRI-a scores improvement achieved with golimumab was maintained at week 104. Improvement in ASspiMRI-a scores correlated with improvement in the recently developed AS disease activity score (ASDAS) and C-reactive protein (CRP) levels but not with other key AS clinical outcomes.
Conclusion Golimumab significantly reduced MRI-detected spinal inflammation of AS; improvements were sustained to week 104 and correlated with improvement in ASDAS and CRP.
This paper is freely available online under the BMJ Journals unlocked scheme, see http://ard.bmj.com/info/unlocked.dtl
Statistics from Altmetric.com
Traditionally accepted disease assessments in ankylosing spondylitis (AS) primarily comprise patient-reported subjective measures, including pain, morning stiffness and fatigue. Objective measures of inflammatory activity, such as C-reactive protein (CRP) or erythrocyte sedimentation rate, may not be elevated in active AS.1
Recent randomised, placebo-controlled studies have shown that MRI can objectively assess the effect of AS treatment interventions. In the largest imaging study yet (n=279), infliximab nearly eliminated MRI-detected spinal inflammation after 24 weeks of therapy.2 Adalimumab has also effected sustained improvement in MRI-detected inflammation in the spine and sacroiliac joints of AS patients.3 Smaller controlled4,–,6 and open-label7 studies have also included MRI, some with data through 2 years of therapy.
In the GO-RAISE trial, patients with active AS showed significant and sustained improvement in signs and symptoms with golimumab treatment.8 9 Here we report findings derived from a GO-RAISE MRI substudy.
Methods
Details of the GO-RAISE study have been reported.8 Adult patients with AS for 3 months or longer, Bath AS disease activity index (BASDAI) score of 4 or greater (0–10-point scale), spinal pain assessment score of 4 or greater on a 0–10 cm visual analogue scale (VAS), and inadequate response to current/previous non-steroidal anti-inflammatory drugs or disease-modifying drugs were randomly assigned (1:1.8:1.8) to placebo, golimumab 50 mg, or golimumab 100 mg injections every 4 weeks. Patients could continue stable methotrexate, sulfasalazine, hydroxychloroquine, corticosteroids and non-steroidal anti-inflammatory drug treatment.
At week 16, patients achieving less than a 20% improvement from baseline in the total back pain and morning stiffness scores entered double-blind early escape: patients randomly assigned to placebo received golimumab 50 mg, patients randomly assigned to golimumab 50 mg received 100 mg, and patients randomly assigned to golimumab 100 mg did not change therapy. At week 24, patients still receiving placebo crossed over to golimumab 50 mg.
The protocol was reviewed and approved by each site's institutional review board or independent ethics committee. All patients provided written informed consent. The MRI substudy was conducted at 10 (of 57) sites with the capability and willingness to participate. All patients enrolled at participating substudy sites were included in the substudy.
Serial spine MRI scans of the cervical, thoracic and lumbar spine in the sagittal plane were acquired with the patient in the supine position using 1.5 Tesla scanners and phase array spine or quadrature coils. Imaging sequences were T1 sagittal, turbo spin echo or fast spin echo without fat suppression, and short tau inversion recovery (STIR) sagittal (table 1).
MRI protocol for sagittal cervical, thoracic and lumbar spine
Images were acquired at baseline (within 4 weeks of initial study agent administration) and within 1 week of the scheduled week 14 and week 104 visits. Seven of 98 (7.1%) MRI substudy patients had baseline MRI images obtained outside of the visit window that were excluded from analyses.
Two qualified, experienced independent readers, blinded to treatment information, patient identity and chronology of the images, scored each sequence. All images were scored by each reader; reader scores for each image were averaged. A randomly selected 10% sample of MRI substudy patients had images re-scored by each reader 3 weeks or more after the initial scoring by that reader.
All 23 vertebral units (VU; C2–S1) were to be evaluated and scored for activity, ie, hyperintense bone marrow lesions (‘bone marrow oedema’) by STIR, and for erosions using the AS spine MRI-activity (ASspiMRI-a) score.5 10 A VU was defined as the area between two virtual horizontal lines through the middle of two adjacent vertebrae. Readers first assessed technical adequacy, or image quality, of the overall spinal segment (cervical, thoracic, lumbar) as optimal, readable but not optimal, or not readable and then scored each evaluable VU as follows: 0 (no bone marrow oedema/erosions), 1 (minor bone marrow oedema involving ≤25% of the VU, no erosions), 2 (moderate bone marrow oedema involving >25% but ≤50% of the VU, no erosions), 3 (major bone marrow oedema involving >50% of the VU, no erosions), 4 (minor erosion involving ≤25% of the VU with minor bone marrow oedema), 5 (moderate erosion involving >25% but ≤50% of the VU with bone marrow oedema), or 6 (major erosion involving >50% of the VU with bone marrow oedema). An unevaluable VU was designated ‘U’ (unable to score). The total ASspiMRI-a score range is 0–138. If one reader's score was missing, the other reader's score was used. If both readers' scores were missing, patient data were considered missing. Only patients with baseline and one or more postbaseline score were included in the analysis. If a week 14 or week 104 score was missing, the score was imputed according to prespecified methodology wherein the 75th percentile score for all patients at that time point was employed.
For the week 104 analysis, patients who entered early escape were included with their originally randomised group without the early escape imputation rule applied. For calculation of percentage change from baseline, if the baseline score was 0 and the postbaseline score was not 0, then the baseline score was set to 0.1. If both baseline and postbaseline scores were 0, then the percentage change from baseline was considered to be 0. Probability plots were constructed using observed data.
After adjusting for the baseline ASspiMRI-a score and treatment group, multivariate regression analysis was employed to assess the relationship between ASspiMRI-a scores and clinical response (ie, proportion of patients achieving ≥20% improvement in Assessment of AS International Working Group Criteria; ASAS20).11 Spearman correlation coefficients were determined to assess relationships between ASspiMRI-a scores and other clinical measures, including BASDAI,12 AS disease activity score (ASDAS),13 physical function assessed by the Bath AS functional index (BASFI),14 total back pain (10-cm VAS) and morning stiffness (10-cm VAS), and also for the relationship between ASspiMRI-a scores and serum CRP, an independent, objective measure of inflammation.
Treatment group differences in the changes from baseline to week 14 were determined using two-sided analysis of variance (ANOVA) on the van der Waerden normal scores (α=0.05). Because of a disparity among treatment groups in baseline ASspiMRI-a scores, a post-hoc ANOVA adjusted for the baseline ASspiMRI-a score. No statistical comparisons were conducted at week 104 because all patients received golimumab from week 24 onward.
To assess the correlation and reliability of the ASspiMRI-a scores at weeks 0, 14 and 104, 10% of images were re-read by each reader for the determination of inter-reader reliability coefficients, reflecting the correlation between scores obtained for the same image by the two different readers at approximately the same time, and read-re-read reliability coefficients, reflecting the correlation between scores obtained for the same image by the same reader at two different times.15 The smallest detectable change in ASspiMRI-a scores at weeks 14 and 104 was determined.
Results
Baseline demographics and disease characteristics
Baseline characteristics of the 98 MRI substudy participants are shown in table 2, along with baseline characteristics of the total study population. In the overall study population, patients in the placebo group were older, had longer disease duration, were more likely to demonstrate human leucocyte antigen B27 positivity, and were more likely to have a history of uveitis versus the golimumab groups.8 The median baseline CRP concentration was also higher in the placebo than golimumab groups. These differences were also evident and somewhat more pronounced in the substudy population. Despite these differences, the substudy population was generally balanced for most characteristics of moderately to severely active AS at baseline and representative of the total study population.
Baseline demographics and disease characteristics for patients in the MRI substudy and the study population overall
MRI activity scores
Eighty-six (87.8%) of the 98 MRI substudy patients had a baseline MRI obtained within the visit window and also had one or more postbaseline MRI image. At weeks 14 and 104, 7.0% (6/86) and 23.3% (20/86) of these patients, respectively, had ASspiMRI-a scores imputed due to missing data (see Methods section). When spinal segment (cervical, lumbar, thoracic) image quality was assessed, 55.4% (412/744) of the segments had one but not both readers designate the quality as ‘readable but not optimal’. Such images still yielded scoreable VU images unless a VU was considered unevaluable for activity scoring. When assessing image quality in individual VU, in 1.3% (77/5711) of VU one but not both readers considered it unevaluable for activity scoring.
The weighted κ coefficient of the inter-reader agreement for ASspiMRI-a scoring was 0.44. The inter-reader reliability coefficients and read-re-read reliability coefficients for ASspiMRI-a scores were 0.54 and 0.78, respectively, at week 0; 0.42 and 0.74, respectively, at week 14; and 0.57 and 0.86, respectively, at week 104. The smallest detectable changes in ASspiMRI-a scores were 6.64 and 2.57 at weeks 14 and 104, respectively.
Baseline ASspiMRI-a scores were lower in the golimumab 100 mg group versus the placebo and golimumab 50 mg groups (table 3). As per the planned analysis, the improvement in ASspiMRI-a score at week 14 was significantly greater in the golimumab 50 mg than the placebo groups (p=0.047), while the difference between golimumab 100 mg and placebo was not statistically significant (p=0.14). In the post-hoc ANOVA that adjusted for disparities in baseline ASspiMRI-a scores, improvements in ASspiMRI-a scores in the golimumab 50 mg and 100 mg groups were significantly greater versus placebo (p=0.011 and p=0.002, respectively; table 3). The median percentage improvements in the ASspiMRI-a score from baseline to week 14 were significantly greater in the golimumab 50 mg and 100 mg groups versus placebo (p=0.032 and p=0.025, respectively; table 3).
Baseline and changes from baseline to weeks 14 and 104 in ASspiMRI-a score
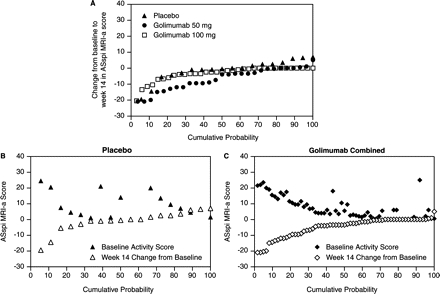
As illustrated in a probability plot (figure 1A), greater improvement/less worsening in spinal inflammation was observed at week 14 in golimumab-treated patients. Figures 1B, C illustrate the relationship between changes from baseline to week 14 and the corresponding baseline ASspiMRI-a score for each patient. In the combined golimumab group plot (figure 1C), points for baseline score and change scores generally mirror each other bisected by a virtual line that is close to 0, indicating nearly complete resolution of MRI-detected spinal inflammation. Conversely, changes in the placebo group were independent of baseline activity, with the exception of two patients with high baseline ASspiMRI-a scores (>20 points), each of whom showed nearly complete reduction in inflammatory activity at week 14.
Panel A: Cumulative probability of changes in ASspiMRI-a scores from baseline to week 14 for each treatment group. Each data point represents the change from baseline for an individual patient. Panels B and C: Double probability plots for the placebo and combined golimumab groups, respectively, showing the baseline ASspiMRI-a score plotted on top of the corresponding change from baseline to week 14 for each individual patient.
In the golimumab groups, improvements (absolute/percentage change) in week 14 ASspiMRI-a scores were maintained to week 104 (table 3). Because all patients in the placebo group either entered early escape (week 16) or crossed over to golimumab 50 mg (week 24), median percentage improvements from baseline to week 104 in the ASspiMRI-a score were similar to those observed in the golimumab groups. Representative images showing MRI-detected improvement in a golimumab-treated patient (50 mg→100 mg) are provided in figure 2.
Magnetic resonance images of the cervical (C) and thoracic (T) spine at baseline (Panel A), week 14 (Panel B) and week 104 (Panel C) of a patient who received golimumab 50 mg followed by early escape at week 16 to golimumab 100 mg. These sagittal Short Tau Inversion Recovery (STIR) images show active lesions at multiple vertebral units, particularly at C7/T1 and T6/T7 at baseline (Panel A). The activity in the spine was markedly decreased at week 14 (Panel B) and resolved at nearly all levels at week 104 (Panel C). Note: Series of consecutive images were evaluated; the images displayed here are representative but not exhaustive.
Among patients with a baseline ASspiMRI-a score greater than 1, 54.2% of patients in the combined golimumab group showed no activity at week 14 compared with 20.0% of patients in the placebo group (p=0.0205; table 3). By week 104, approximately 1.5 years after placebo patients crossed over to golimumab, the percentages of patients with no inflammation were comparable between the patient groups (61.9% and 66.7%, respectively; p=0.76).
By concordance of both readers, 14 (21.5%) of the 65 patients with available MRI data had baseline inflammation on the MRI that persisted at week 104, although not necessarily in the same areas. When compared with the entire MRI substudy population, the baseline characteristics in these patients were not different and no predictive factors were evident.
Relationships between MRI activity scores and clinical measures
Achievement of ASAS20 response was not significantly associated with change from baseline in ASspiMRI-a score (multiple regression coefficient (R)=0.62, p=0.17; data not shown). In the combined golimumab group, median (IQR) changes from baseline to week 14 in the ASspiMRI-a score were 0.0 (−11.5, 0.5) and −3.5 (−7.0, −0.5) in week 14 ASAS20 non-responders and responders, respectively (p=0.11; data not shown).
Conversely, there was a significant positive correlation between changes at week 14 in ASspiMRI-a and ASDAS in the combined golimumab group (Spearman correlation coefficient (r)=0.35, p=0.004) but not in the placebo group (r=0.39, p=0.09). Also, baseline ASDAS significantly correlated with the change in ASspiMRI-a at both week 14 (r=−0.30, p=0.015) and week 104 (r=−0.33, p=0.007) in the combined golimumab group.
We failed to find consistent correlations between ASspiMRI-a scores and other key clinical measures, including BASDAI, BASFI, total back pain and morning stiffness, among patients in the combined golimumab group (table 4) or within the placebo group, with the exception of a Spearman correlation coefficient (r) of −0.55 between baseline BASDAI scores and change from baseline to week 104 in ASspiMRI-a score (p=0.014), which is after patients in the placebo group had crossed over to golimumab.
Spearman correlation coefficients showing the correlations between measures of disease activity (BASDAI score) and MRI activity score and between inflammation (CRP level) and MRI activity score for patients in the combined golimumab group
In contrast, strong and highly significant correlations were observed in the combined golimumab group between baseline CRP levels and baseline ASspiMRI-a scores and between baseline CRP levels and changes from baseline to weeks 14 and 104 in ASspiMRI-a scores (table 4), such that higher baseline CRP levels were associated with higher baseline ASspiMRI-a scores and with greater improvement in ASspiMRI-a scores from baseline to weeks 14 and 104. Also, changes from baseline to weeks 14 and 104 in CRP levels significantly correlated with changes from baseline to weeks 14 and 104 in the ASspiMRI-a score. In the placebo group, no correlations between CRP levels and ASspiMRI-a scores were observed (data not shown), except for a Spearman correlation coefficient of 0.70 between the changes from baseline to week 14 in CRP levels and ASspiMRI-a scores (p<0.001).
Discussion
The findings derived from an MRI substudy of the GO-RAISE trial, which evaluated golimumab in the treatment of active AS,8 indicate that golimumab significantly reduced MRI-detected spinal inflammation in patients with AS. These improvements were consistent with the clinical benefits observed in golimumab-treated patients in this study.8 In the planned analysis, the improvement was statistically significant only in the golimumab 50 mg group. However, when the analysis was repeated to adjust for disparities in baseline ASspiMRI-a scores (golimumab 100 mg group had substantially lower scores), the subcutaneous administration of both golimumab 50 mg and 100 mg yielded statistically significantly greater improvements in ASspiMRI-a scores versus placebo. Results of follow-up spinal MRI performed at week 104 indicated that these reductions in inflammation were maintained through 2 years of treatment.
Although the magnitude of change from baseline in MRI-detected inflammation appeared to be numerically greater in the 50 mg than the 100 mg group, the median improvement was similar in both groups when expressed as a percentage change from baseline. A clearly greater percentage reduction in spine inflammation occurred by week 14 with golimumab than with placebo.
This substudy was limited by the relatively small number of patients in each treatment group, which amplified disparities between the treatment groups in disease duration and led to inconsistencies in other important clinical parameters, including baseline ASspiMRI-a scores. Imputation of missing scores for approximately 7% and 23% of patients at weeks 14 and 104, respectively, had the potential to result in the generation of data outliers, which in turn impact mean values in groups of limited sample size. Furthermore, the large number of tests performed for these analyses could have resulted in spuriously significant correlations. An additional study limitation is the only modest level of agreement between the two readers. It is possible that reader accuracy was affected by non-uniform image quality from the different study sites contributing MRI scans to this substudy. Also, the technical adequacy of the spinal segment (cervical, thoracic, lumbar) images, ie, 55.4% of spinal segment images were rated as having a ‘readable but not optimal’ image quality by one of the two readers, may limit the interpretation of our findings. Scoring AS activity at the VU level could also have been impacted by reader discordance, eg, one reader might have scored a VU as containing an extensive diffuse lesion, while the other reader might have viewed the VU as having a high degree of background noise and not as inflamed. However, on a VU basis, only 1.3% of images had one reader score the unit while the other reader considered it unevaluable for scoring. Another possible study limitation relates to the ASspiMRI-a methodology employed, which assesses the presence but not the degree of bone marrow oedema. As it has recently been shown that combined erosion plus bone marrow oedema findings yield enhanced MRI sensitivity in detecting AS,16 future studies may additionally include assessments of the degree of bone marrow oedema. However, whether this will increase the sensitivity to change remains to be demonstrated.
Improvement in ASspiMRI-a scores correlated with improvement in the recently developed ASDAS, a discriminatory instrument for assessing AS activity that includes patient-reported outcomes and CRP levels.13 Results of the correlation analyses also suggest that the greatest improvement in spinal inflammation measured by ASspiMRI-a is likely to occur in patients with higher AS disease activity at baseline, as assessed by ASspiMRI-a, before golimumab treatment. We observed weak or inconsistent correlations between MRI activity scores and disease activity assessed by BASDAI and no correlations between MRI activity scores and other clinical measures of physical function, back pain and morning stiffness. In contrast, significant correlations were observed between ASspiMRI-a scores and CRP concentrations. Although this link is not surprising because both CRP as an acute phase reactant and ASspiMRI-a score as an imaging marker of spinal inflammation can be considered as objective indicators of AS inflammation. Therefore, our data confirm previous reports that changes in disease activity visible with MRI may not correlate with most clinical criteria such as pain, stiffness, function, response to treatment (eg, ASAS20) and metrology indices.3 6 7 However, this is the first investigation to show that the MRI changes do correlate well with the new ASDAS and changes in CRP levels. While these findings suggest that the ASDAS provides a more objective assessment of AS activity than other available clinical measures, we cannot exclude the possibility that the relationship is driven by CRP levels as they are a component of the ASDAS.
In summary, golimumab treatment significantly reduced the MRI-detected spinal inflammation in AS by week 14, and the improvements were maintained to week 104. Improvements in spinal inflammation correlated with improvements in ASDAS and CRP levels, an objective measure of inflammation, but not with various other subjective measures of clinical improvement.
Acknowledgments
The authors thank the patients, investigators and study personnel who made this trial possible. The authors also thank Michelle Perate, MS, of Janssen Biotech, Inc. who helped prepare the manuscript but did not meet the criteria for authorship.
References
Footnotes
-
Funding This study was funded by Centocor Research & Development, Inc. and Merck, Inc. (formerly Schering-Plough Research Institute, Inc.).
-
Competing interests XB belongs to the Spondyloarthritis Immunology Research Alliance (SpIRAL). AB was an employee of Centocor during the time this study was conducted. JB has received consulting fees, speaking fees, and/or honoraria from Centocor, Schering-Plough, Wyeth, Amgen, Abbott, Pfizer and Bristol-Myers Squibb. AAD has received payments for educational lectures, teleconferences and serving on advisory boards for Centocor, a company that may have a commercial interest in the results of this research. This potential conflict of interest has been reviewed and managed by Oregon Health and Science University. KGAH: none declared. RDI has served as a consultant for Abbott, Amgen-Wyeth, Centocor, Merck, Pfizer and Sanofi-Aventis. DvdH has received consulting fees and/or research grants from Abbott, Amgen, AstraZeneca, BMS, Centocor, Chugai, Eli-Lilly, GSK, Merck, Novartis, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB and Wyeth. SX, WX and BH are employees of Centocor and own stock in its parent company (Johnson & Johnson).
-
Ethics approval The protocol was reviewed and approved by each site's institutional review board or independent ethics committee.
-
Patient consent Obtained.
-
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Corrections