Article Text
Abstract
Objectives To develop a preliminary composite psoriatic disease activity index (CPDAI) for psoriasis and psoriatic arthritis.
Methods Five domains were assessed and specific instruments were employed for each domain to determine the extent of domain involvement and the effect of that involvement on quality of life/function. Disease activity for each domain was then graded from 0 to 3 giving a CPDAI range of 0–15. Patient and physician global disease activity measures were also recorded and an independent physician was asked to indicate if treatment change was required. Bivariate correlation analysis was performed. Factor, tree analysis and standardised response means were also calculated.
Results Significant correlation was seen between CPDAI and both patient (r=0.834) and physician (r=0.825) global disease activity assessments (p=0.01). Tree analysis revealed that 96.3% of patients had their treatment changed when CPDAI values were greater than 6; no patient had their treatment changed when CPDAI values were less than 5.
Conclusion CPDAI correlates well with patient and physician global disease activity assessments and is an effective tool that clearly distinguishes those who require a treatment change from those who do not.
Statistics from Altmetric.com
Psoriatic arthritis (PsA) is a distinct inflammatory arthritis that may develop in 5–40% of individuals with psoriasis.1 The heterogeneity of PsA is such that the term ‘psoriatic disease’ has been suggested to reflect more accurately how patients may be affected. In addition, the spectrum of the disease features can vary from mild to very severe adding to the significant challenge to the treating physician in assessing their patients. Patients may present with some or all of these features and it may be difficult to weigh their relative importance, in particular when making decisions regarding therapy. Whereas each of these areas of involvement can be considered separately, in reality there is overlap in their effects on patients, in particular as regards quality of life and functional status. Decisions regarding treatment often depend on the particular focus of the treating physician, that is, dermatologists judge skin activity, rheumatologists judge joint activity but neither may judge the totality of how the patient's health is affected. The development of a composite measure of disease activity, in particular one that includes patient-reported outcomes, should facilitate a more accurate assessment of disease status. Finally, with the advent of more effective treatments for all aspects of psoriatic disease such as biological agents, there is an urgent need both to develop a composite disease activity measure but also a response measure, for the benefit of patients in all dimensions of the disease.2
Preliminary work has been undertaken in developing a more comprehensive disease activity instrument for psoriatic disease. First, the International Group for Research in Psoriasis and Psoriatic Arthritis (GRAPPA) have proposed a grid system for recording mild, moderate and severe disease for the domains of peripheral arthritis, skin disease, spinal disease, enthesitis and dactylitis with the intent of the grid informing decisions for treatment.3 Second, based on the grid system, we have further developed the grid, refined the instruments, and have applied a numerical scale that allows a composite psoriatic disease activity index (CPDAI) to be computed. Individual domain components may also be separately measured. The CPDAI can then be used to develop a disease response measure for testing in randomised controlled trials. Finally, GRAPPA have recently initiated a multicentre prospective study (GRAPA Composite Exercise, GRACE project) in which all the appropriate disease measures are being recorded with a view to refining further a composite activity and a treatment response measure.
In this study, the results of a performance analysis of the CPDAI in a cohort of patients recruited from two rheumatology clinics are presented and compared with both patient and physician global assessments of disease activity. Cut-off scores associated with the need for treatment change are defined. Factor analysis and standardised response means (SRM) were calculated to ensure content validity and to measure responsiveness to change.
Methods
Patients were recruited consecutively from rheumatology outpatient clinics in Dublin and Leeds. The research was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki. Prior approval from the St Vincent's University Hospital ethics committee was achieved. In Leeds ethical approval for collecting all the relevant data, as part of a PsA database, has also been obtained. All patients satisfied the CASPAR4 criteria for a diagnosis of PsA.
Domains and instruments
As proposed by GRAPPA, disease activity was assessed under five domains including joint disease, skin involvement, enthesitis, dactylitis and spinal disease.5 GRAPPA has also recently agreed which instruments best measure some of these domains including the 66/68 swollen and tender joint counts for peripheral joint disease, the health assessment questionnaire (HAQ) physical component score6 as a measure of physical function and the psoriasis area severity index (PASI) for the extent and severity of skin involvement.7 In developing a composite index, for each domain an instrument measuring the extent of involvement and a measure of the effect of that involvement on quality of life/function were included. The list of domains with the instruments used together with the range of values obtained is shown in table 1.
Domains, instruments and range of values
We have chosen domains and instruments that have in general performed well in previous studies and were chosen by GRAPPA members as being essential components of psoriatic disease documentation.8 For dactylitis, a simple dactylitic digit count was applied. For enthesitis, the Leeds enthesitis index (LEI), which was recently validated following a comparison with other entheseal scores, was utilised.9 For spinal involvement, the Bath ankylosing spondylitis disease activity index (BASDAI)10 and the ankylosing spondylitis quality of life (ASQoL),11 which have both been extensively validated in the setting of ankylosing spondylitis (AS), were used. Finally, the dermatology life quality index (DLQI)12 was used as a measure of the impact of skin disease.
The severity grading or cut-offs for mild, moderate and severe were chosen largely based on literature review, and they are similar to those proposed by GRAPPA in their previous publication on treatment guidelines. It has been shown that significant polyarticular inflammation (more than four joints involved) predicts a poor outcome in terms of joint damage. Moreover, Kane et al13 have shown that PsA patients are more likely to be in remission if they have oligo-articular (≤4) involvement from the onset. Based largely on the literature on AS it has been shown that a BASDAI of greater than 4 accurately reflects active disease. Furthermore, a treatment response again based on definition and response in AS is defined as a 50% relative change or absolute change of 2.14 In relation to skin disease, many sources consider greater than 10% PASI to be indicative of moderate to severe skin involvement15 and less than 10% PASI is considered as mild. Psoriasis results in significant impact on quality of life and the DLQI has been shown to detect meaningful changes over time. Generally a DLQI greater than 10 depicts severe impact on quality of life of patients.16 As yet cut-offs for severe dacylitic or entheseal disease have not been validated, but we have used the same cut-offs in this study as previously proposed by GRAPPA.
Composite psoriatic disease activity index
Using the above instruments and ranges of values, disease activity under each domain was graded as none, mild, moderate and severe (0–3) (table 2), giving a range attainable CPDAI scores of between 0 and 15.
CPDAI score total 0–15
As alternative measures of disease activity, a patient and a physician global assessment of disease activity was recorded each using a 10 cm visual analogue scale. In order to assess the ability of the CPDAI to predict a change in treatment, an independent physician (OF) was also asked to record whether the overall disease activity was such that a treatment change was required. Finally, instruments related to spinal disease were recorded only in those patients with symptoms of inflammatory-type back pain.
Statistical analysis
Data were analysed using SPSS statistical software version 17. Median values for each of the instruments were calculated. As the data were non-parametric, the Spearman rank correlation coefficient was used to calculate correlation between the CPDAI and both patient and physician global disease activity measures. A tree analysis was performed to help derive a treatment decision tool based on the CPDAI values. Factor analysis with varimax rotation was used to extract components contributing to maximum variability in the data. All tests were two-tailed and p<0.05 was considered to be significant. Furthermore, the responsiveness of the CPDAI was calculated by using SRM.
Results
Patient cohort
Baseline characteristics were calculated for 117 consecutive PsA patients collected over a period of 12 months (Dublin 93, Leeds 24). The cohort included 52 men with a median age at baseline of 47 years (IQR 18). The median duration of the articular symptoms and of psoriasis was 10 months and 14 years, respectively. The time between psoriasis and PsA diagnoses was 5 years.
Disease variables
The median values with ranges for each of the disease instruments are shown in table 3.
Baseline disease variables (median with range) and calculated CPDAI in the 117 psoriatic arthritis patients
Of note, the median PASI scores in this cohort are low at 1.6, reflective of patients attending a rheumatology clinic, and many already established on effective treatment at the time of assessment.
CPDAI correlation with patient and physician global disease activity assessments
The Spearman rank correlation coefficient was used to demonstrate the correlation of the CPDAI with patient and physician global disease activity assessments (table 4).
CPDAI correlation with physician and patient global assessments
As can be seen, there was a highly significant correlation between all three variables, in particular between the CPDAI and physician global assessment, giving the disease activity measure construct validity.
Predicting treatment change
The median CPDAI score in patients thought to require a treatment change by an independent physician was 9.0; the corresponding score consistent with no change being required was 3 (p<0.05). (figure 1).
Box plot representation of the median composite psoriatic disease activity index (CPDAI) scores in patients in whom treatment change was thought to be indicated (n=40) by an independent physician compared with the value in those in whom treatment change was thought not to be required (n=77).
Tree analysis
The results of the tree analysis are given in figure 2. As can be seen, treatment change was thought to be required in 36.3% of patients. No treatment change was made if the CPDAI values were less than 4; on the other hand, 96.4% of patients had their treatment changed if their CPDAI values were greater than 6. CPDAI values of 5 or 6 accounting for 19.8% of the cohort were deemed indeterminate. The CPDAI accurately identified over 80% of patients as either requiring or not requiring a change in treatment. Therefore, the tree analysis helps to distinguish between states of interest, captures the concept of classification and satisfies the discrimination aspect of the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) filter (figure 2).
Tree analysis an objective way of establishing the discriminate ability of the composite psoriatic disease activity index (CPDAI). COT, change of treatment.
Factor analysis
In order to extract components contributing to maximum variability in the data, a factor analysis was used to produce a solution using principal components extraction, which was then rotated (varimax rotation) for ease of interpretation. Previous log transformation of the data was undertaken to achieve meaningful results. As is shown in table 5, the first rotated factor is most highly correlated with the swollen joint count and dactylitic digit count; moreover, these variables are not correlated with the other three components. The second rotated factor correlates with the PASI and DLQI, the third with the LEI and tender joint count, and the final factor is highly correlated with the HAQ. This process ensures the content validity of the tool by incorporating variables that capture maximum variability in the data.
Factor analysis showing the amount of variance in the original variables accounted for by each component
Assessment of spinal involvement
ASQoL and BASDAI were calculated in 41 patients (35.3%) who satisfied the CASPAR4 criteria and also had symptoms of inflammatory-type back pain for more than 3 months. Of these patients, 39.34% had radiological evidence of sacroiliitis. In those patients undergoing treatment change (n=26) the median values for calculated CPDAI, BASDAI and ASQoL were 9.0, 6.35 (0–10) and 13 (0–18), respectively.
Response to treatment change
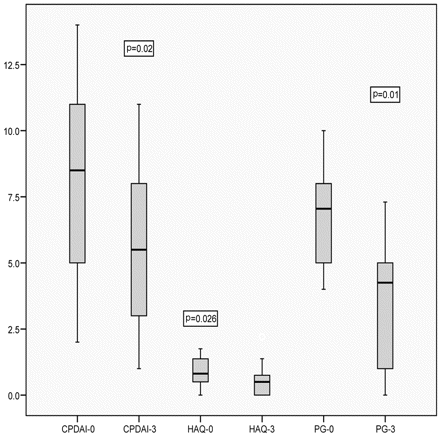
In total, 25 patients were started on a new treatment. At 3 months of follow-up, the median CPDAI decreased from 8.5 to 5.5 (p=0.02), the median HAQ decreased from 0.85 to 0.57 (p=0.026) and the median patient global decreased from 6.9 to 3.4 (p=0.001) (figure 3). Further assessment of the CPDAI to detect responsiveness was obtained by calculating SRM. The SRM for the CPDAI was calculated to be 0.60.
The change in the composite psoriatic disease activity index (CPDAI), health assessment questionnaire (HAQ) and patient global (PG) from the baseline over 3 months in patients started on new treatment (n=25).
Criterion validity
We checked the criterion validity of the CPDAI by comparison with the disease activity score using 28 joint counts (DAS28). The CPDAI correlated significantly with the DAS28 (r=0.849).
Discussion
In this study, we have endeavoured to develop a composite measure of PsA disease activity. The need for such a tool, which adequately assesses this complex and heterogeneous disease in all its totality, using specific domains and incorporating quality of life and functional measures has been highlighted. The CPDAI calculates a numerical score and by using a tree analysis it was possible to establish cut-off values for predicting a change of treatment. Our study has demonstrated the CPDAI to be a good discriminant tool, able to distinguish between patients requiring a treatment change from those who do not. It correlates well with patient and physician global assessments, further adding to its construct validity. In an initial analysis, the CPDAI has shown good ability to detect meaningful responsiveness to change over time (SRM=0.60) and has also demonstrated good criterion validity in comparison analysis with the DAS28.
The tool is feasible and easy to perform. On average it takes 7–8 min to perform the complete examination and calculate the score and is therefore quite practical in busy clinical settings.
Disease activity was assessed across five domains using specific tools and instruments, many of which have been validated by GRAPPA and OMERACT. This adds to satisfying the OMERACT truth filter by establishing face content and validity. A 66 tender and 68 swollen joint count was used in order to assess the joints adequately, as it has previously demonstrated good inter and intra-observer variability in PsA.17 This joint count has also become the standard measure in PsA, partly because of its inclusion in key composite criteria including those proposed by GRAPPA.18
Assessment of enthesitis is an integral part of the core set of domains proposed by GRAPPA and OMERACT. A number of different instruments has been proposed, some of them having been borrowed from AS.19 These include the Newcastle enthesitis index (NEI) developed by Mander, the Maastricht AS enthesitis score (MASes) and the spondyloarthritis research consortium of Canada (SPARCC) enthesitis index. The NEI involves assessment of 66 sites and it is considered to be too subjective and time-consuming for clinical practice. The MASes19 evaluates 13 sites and the SPARCC proposed by the Canadian group evaluates enthesitis at eight sites.20 Recently, Healy and Helliwell9 proposed a new index, the LEI, specifically for PsA following an iterative process of data reduction and comparison with other instruments. The LEI is simple and easy to perform and shows good responsiveness and effect size (1.19) compared with other measures.
Dactylitis is a hallmark clinical feature of PsA. It is present in approximately 50% of patients at some point in the course of their disease and, in a study conducted in Dublin, the prevalence of dactylitis in an early arthritis clinic was 30%.21 Dactylitis involves swelling of an entire digit affecting the joint and the tendon with a major component being tenosynovitis.22 It is an important prognostic indicator of the severity of PsA, as dactylitic digits show more radiographic progression of damage.23 In this study, dactylitis was assessed by using a simple digit count. The examining physician was asked to record the presence of swelling and/or tenderness in the involved digits. The IMPACT 1 and IMPACT 2 trials have shown this measure to have good sensitivity to change.24
Axial involvement in PsA is quite heterogeneous in its expression and is distinct from AS, characterised by less pain, lower sacroiliac grade and less syndesmophyte formation on radiographs.25 The ASAS working group recommended the use of the BASDAI as a clinical outcome measure in AS. The recently conducted INSPIRE trial demonstrated that instruments used for the assessment for AS are also applicable to PsA. In a study by Fernández-Sueiro et al,26 the BASDAI scores were significantly higher in axial PsA patients compared with peripheral arthritis PsA patients, and were similar to those of primary AS patients, which suggests that the BASDAI may be a useful clinical tool for evaluating spinal inflammation in patients with PsA. We used the ASQoL as a specific health-related quality of life measure in these patients. The ASQoL, although not validated in PsA, has been shown to detect meaningful changes over time with treatment in AS patients.27
The current gold standard for the assessment of extensive psoriasis has been the PASI.7 The PASI score has been widely used by regulatory authorities as well as in numerous clinical trials to date. A major criticism of the PASI has been its inability to detect sensitivity to change in patients with a lower range of skin involvement (for example with a PASI score of <3).2
We acknowledge and recognise certain limitations in this study. For example, criticism might be raised about the very low median PASI score in this cohort. All the patients were recruited from rheumatology outpatient departments and not from dermatology clinics, where undoubtedly the skin scores would have been more impressive. The CPDAI needs further assessment in larger cohorts of patients, particularly those with extensive skin involvement, to assess its ability to measure psoriatic disease activity in its totality. It is currently being assessed in dermatology outpatients for this purpose.
In our composite model, the HAQ is employed in three domains, and we acknowledge that this may cause some redundancy in the system. Physicians are frequently faced with situations in which disease extent can be fairly minimal but nonetheless may result in significant impact on patients' function and quality of life. It is certainly possible that the HAQ will reflect disease activity or function (damage) in at least three of the domains, but currently there are no available instruments that allow us to separate individual domain involvement and its effect on patients' function. In order to overcome this problem, future study instruments such as visual analogue scale scores might be developed that reflect involvement at entheseal and dactylitis sites. These instruments can then be incorporated into a future version of the CPDAI.
To date, we have not correlated the CPDAI with structural damage, and to ensure criterion validity of the CPDAI this is the subject of an ongoing study.
Another limitation of this study was that spinal involvement was assessed only in those patients with features of inflammatory back pain for the past 3 months. It would be important to assess spinal involvement in all patients and to determine its potential contribution towards not only the CPDAI but also in predicting a change of treatment.
This study represents a first attempt to develop a composite measure of psoriatic disease activity. Other composite activity or response measures developed for PsA, such as the psoriatic arthritis response criteria,28 or the disease activity index for reactive arthritis (disease activity index for psoriatic arthritis),29 30 have focused only on joint disease and have not included measures of other key disease domains such as spinal or skin involvement. The CPDAI proposed here is therefore different in that it can on the one hand allow for a domain to be separately evaluated, but at the same time a composite score reflecting all of the involved domains can be calculated. We have shown that the CPDAI begins to satisfy the three components of the OMERACT filter, that is, truth, discrimination and feasibility. The CPDAI needs further construct and discriminant validity by application in randomised controlled trials and longitudinal observational studies. Prospective validation studies are currently underway as part of a larger multicentre study organised by the GRAPPA group, as well as a study comparing the CPDAI with ultrasonography as a more objective measure of disease activity.
Acknowledgments
The authors would like to thank Dr Tom O'Hara (Trinity College Dublin) for his statistical support.
References
Footnotes
-
Funding AM's University College Dublin Newman Fellowship was supported by an unrestricted educational grant from Abbott (Ireland) Ltd. LCC is supported by the Arthritis Research Campaign as an ARC Clinical Research Fellow.
-
Competing interests None.
-
Ethics approval This study was conducted with the approval of the ethics committees of St Vincents University Hospital Dublin and the University of Leeds.
-
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Miscellaneous