Article Text
Abstract
Objective To examine the association of skin thickness progression rate (STPR) with mortality, and as a predictor of future internal organ involvement in an inception cohort of diffuse cutaneous systemic sclerosis (SSc) patients.
Methods Diffuse cutaneous SSc patients older than 16 years of age evaluated at the University of Pittsburgh within 2 years of the first evidence of skin thickening between 1980 and 2005 were eligible. The authors calculated the STPR on these patients, and examined the relationship of this variable to the development of early internal organ involvement and short-term mortality using logistic regression.
Results 826 patients were included in the analysis. Patients with a rapid STPR experienced significantly reduced short-term survival at 1 and 2 years from the time of first Pittsburgh evaluation (p=0.002). Patients with a rapid STPR were more likely to develop renal crisis within 1–2 years of follow-up. Rapid STPR was found to be an independent predictor of both mortality (OR 1.72; 95% CI 1.13 to 2.62; p=0.01) and ‘renal crisis’ (OR 2.05, 95% CI 1.10 to 3.85; p=0.02) within 2 years from first evaluation.
Conclusion The STPR is an easy measure to perform at the time of initial evaluation for identifying those diffuse cutaneous SSc patients who are at increased risk of mortality and the development of renal crisis during the following 2 years.
Statistics from Altmetric.com
Systemic sclerosis (SSc) is a multisystem autoimmune disease characterised by inflammation and excessive deposition of extracellular matrix in the skin and internal organs. Its clinical course can range from a relatively benign condition, with only skin and peripheral vascular involvement, to a rapidly progressive disease affecting one or more internal organs. Little has been published to assist managing physicians in identifying patients who are at high risk of serious visceral involvement or death early in their disease course. In 1984, we introduced the notion that a rapid increase in skin thickening was a risk factor for the development of scleroderma renal crisis (SRC), but did not suggest a quantitative method for expressing a rate of skin thickness increase.1 Clinical trials have focused on evaluating the results of drug therapy, but have used a variety of entry criteria that may have created heterogeneous groups at different risks of particular outcomes, thereby making it difficult to assess true clinical outcomes and response to therapy. A clinical measurement tool to identify high-risk groups for mortality and early internal organ involvement at the first patient visit would be helpful for clinical care, and would enhance clinical trial design and conduct. We sought to develop such a clinical measurement tool.
The purpose of the study was to examine the skin thickness progression rate (STPR), obtained by history and physical examination, as a predictor of internal organ involvement and mortality outcome in an inception cohort of SSc patients with diffuse cutaneous involvement.
Methods
Patient selection
All patients undergoing an initial evaluation at the University of Pittsburgh Scleroderma Clinic between 1980 and 2005 who were 16 years of age or older at the time of first physician diagnosis of SSc were eligible. We included only patients with diffuse skin involvement evident at the time of initial evaluation. As our data have been prospectively collected, we created an inception cohort by requiring that the length of time from the onset of skin thickening to the first visit should be less than 2 years. We excluded those who were not US citizens or had moved out of the USA, as accurate follow-up vital status information could not be obtained for these patients.
Clinical information
Clinical information on all patient visits to our clinic has been recorded prospectively on standardised data collection forms since 1980. We included clinical symptoms, complete physical examination with modified Rodnan total skin score (mRSS) and palpable tendon or bursal friction rubs, objective studies of internal organ involvement and estimated date of onset for the involvement of each internal organ. All mRSS were performed by one of three experienced attending physicians. Our referral area was defined as patient residence at the time of the first visit within 100 miles of Pittsburgh.
Skin thickness progression rate
The onset of skin thickening was defined as the first time (month and year) that the patient's fingers became swollen and never again returned to normal size. We used the patient's history to make this judgement, supported by referring physicians' medical records. Skin thickness progression rate (STPR) was defined as the mRSS at the first visit divided by the duration of skin thickening (in years) by patient report. For example, if the mRSS was 24 at the time of the first visit, and the duration of skin thickness is 8 months, then the STPR for this patient was 24/0.67 years, or 36 per year.
Autoantibody identification
Anti-topoisomerase I antibodies were determined by immunodiffusion. Anti-RNA polymerase III, anti-PM-Scl, anti-Ku, anti-U1RNP, anti-U3RNP, anti-U11/U12RNP and anti-Th/To antibodies were determined by protein immunoprecipitation.
Definitions of organ system involvement
We defined organ system involvement based on the presence of clinical features observed during the course of scleroderma and not attributable to other conditions. Renal involvement was defined as clinical evidence of SRC including new onset hypertension (blood pressure >140/90) and/or a rise in serum creatinine, with or without evidence of microangiopathic haemolytic anaemia. Cardiac involvement included one or more of the following: (1) pericarditis, defined as pericardial pain in combination with EKG findings, pericardial rub or a confirmed pericardial effusion by CT or echocardiogram; or (2) myocarditis or cardiomyopathy defined as left ventricular ejection fraction less than 45% not attributable to pulmonary hypertension or left heart disease, with or without cardiac enzyme abnormalities; or (3) conduction system abnormalities, defined as arrhythmia requiring treatment or complete heart block. Pulmonary involvement included evidence of pulmonary fibrosis or ground glass opacities on chest radiograph or high resolution CT scan or forced vital capacity (FVC) less than 70% with a forced expiratory volume/FVC ratio greater than 80%. Pulmonary arterial hypertension was defined as estimated pulmonary arterial systolic pressure of 40 mm Hg or greater on transthoracic echocardiogram or mean pulmonary artery pressure greater than 25 mm Hg on cardiac catheterisation. Gastrointestinal involvement required objective evidence of oesophageal dysmotility on cine-oesophagram or manometry, oesophageal stricture, small bowel hypomotility or dilation on imaging, the use of antibiotics for small bowel bacterial overgrowth, malabsorption syndrome (physician judgement), the use of parenteral nutrition, or wide-mouthed colonic sacculations.
Dates of organ system involvement were determined by the date of first symptom attributable to this organ system, provided that the symptom was followed by objective evidence of involvement, or the objective evidence itself, whichever came first. These symptoms included elevated blood pressure or an increase in serum creatinine for SRC, dyspnoea for pulmonary involvement or cardiac involvement, pericardial pain for cardiac involvement and heartburn or distal dysphagia for gastrointestinal involvement. For example, if a patient developed heartburn in July 1983, and had an abnormal cine-oesophagram in October 1983, the date of gastrointestinal involvement was recorded as July 1983.
Outcome measurements
The primary outcome of interest was the development of serious internal organ involvement within 2 years of the first Pittsburgh visit. We assessed the development of SRC, pulmonary, gastrointestinal and cardiac involvement separately. The secondary outcome was mortality from the time of the first Pittsburgh visit. Vital status as of 31 December 2009 was determined through the Social Security Death Index.
Statistical analysis
Baseline characteristics were calculated by measures of central tendency and proportions. Survival differences between STPR groups were determined using the Kaplan–Meier method. We examined predictors of survival from both the onset of skin thickening and the first visit. χ2 Tests were used to compare the frequency of internal organ involvement (renal, cardiac, pulmonary and gastrointestinal) between STPR subgroups. Logistic regression analysis was used to evaluate predictors of the development of SRC, cardiac, pulmonary and gastrointestinal involvement. Univariable analysis was initially performed, and variables with a p value of less than 0.20 were selected for stepwise multivariable logistic regression. Two-way interactions were adjusted for in the final models.
Results
Eight hundred and thirty-three patients satisfied the entry criteria and lived within the USA. Of these, 826 patients had data available to calculate a STPR. The mean age at the first visit to Pittsburgh was 49 years (range 17–81 years). As expected, the population had a 3:1 ratio of women (75%) to men (25%) and was predominantly Caucasian (90%). Forty-six per cent of the 823 patients had anti-RNA polymerase III antibody, 25% anti-topoisomerase I antibody, 3% anti-U3 RNP antibody, 2% U1-RNP antibody, 1% each of U11/U12-RNP, anti-Ku, PM-Scl and anti-centromere antibody. Twenty per cent did not have a SSc antibody recognised or did not have complete antibody testing performed. The median length of time from patient-reported onset of skin thickening to the first visit was 0.72 years (interquartile range (IQR) 0.50–1.08), from the onset of the first SSc-associated symptom 1.10 years (IQR 0.71–1.67) and from the first non-Raynaud symptom 0.91 years (IQR 0.61–1.39). The mean mRSS at the first visit was 26 (±11.4). The median STPR was 34 per year, with a range of 2–358 per year. Due to this wide variability, the STPR was tertiled for analysis. This corresponded to a STPR score of less than 25 per year for slow STPR, 25–44 per year for intermediate STPR, and over 45 per year for rapid STPR.
We compared the baseline characteristics between the three STPR groups (table 1). There was no difference in age, gender or race between the three groups. Patients with a rapid STPR had a higher median total skin score (34) and had a shorter interval between the onset of skin thickening and their first Pittsburgh visit when compared with the other two groups. There was a significantly greater proportion of anti-RNA polymerase III antibody patients in the rapid STPR group compared with the intermediate and slow group (p≤0.0001), with the greatest percentage of patients with other scleroderma autoantibodies in the slow STPR group (33%; p<0.0001). There was no difference in the proportion of patients having had renal crisis, cardiac, pulmonary or gastrointestinal involvement at the time of the first visit.
Baseline characteristics by STPR group
Survival
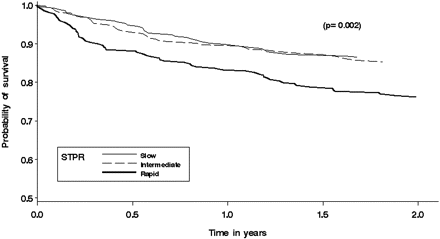
Overall, 51% of all patients were alive as of 31 December 2009. We examined 1 and 2-year mortality by STPR group from two time points, the onset of skin thickening and the first visit. The pattern of results was similar for both starting times. The proportion of patients in the rapid STPR group dying within 1 or 2 years from the first visit was higher at 1 year (17%; p=0.02) and 2 years (24%; p=0.002) and at 2 years compared with the other groups, as depicted in figure 1. The increased proportion of deaths in the rapid STPR group persists at 5 years of follow-up, with 35% of rapid STPR patients deceased, compared with 25% of the intermediate and 22% of the slow STPR patients (p=0.002). Despite the fact that patients with a slow STPR are seen later in their disease, their short-term mortality from the time of the first visit is lower (10.0% at 1 year, 13% at 2 years). There was a significant difference in survival at 2 years from both the onset of skin thickening and the first visit (figure 1) in the rapid STPR group compared with the intermediate and slow STPR groups.
Survival by skin thickness progression rate (STPR) group at 2 years from the first visit (p<0.0002).
A multivariable analysis was performed to evaluate independent predictors of 1 and 2-year mortality from the time of the first visit. We used information obtained at that visit including demographic information, the presence of internal organ involvement, autoantibody, skin score, STPR group and tendon friction rubs. The results for 2-year mortality are shown in table 2. Significant independent predictors of mortality at 1 year include age greater than 55 years, the presence of cardiac involvement, palpable tendon friction rubs and rapid STPR. At 1 year similar results were found, with the exception that gastrointestinal involvement also predicted 1-year mortality. In addition to STPR we also used the skin score, and the categorical variable skin score greater than 20. Neither of these measures was significant independent predictors of mortality at 1 or 2 years in multivariable analysis.
Multivariate analysis of first visit variables predictive of mortality at 2 years
Development of early internal organ involvement
We examined associations of STPR with the development of early internal organ involvement. At the time of the first visit to Pittsburgh, a tertiary referral centre, many patients had already developed internal organ involvement. The presence of internal organ involvement before or at the first Pittsburgh visit was compared between STPR groups. There was no significant difference in SRC, although 12% of patients in the rapid STPR group had had SRC, compared with 9% in both the intermediate and slow groups, respectively (p=0.03). There was no difference in the proportion of patients with cardiac, pulmonary or gastrointestinal involvement at the first visit. We then sought to assess if STPR predicted the development of new organ involvement within 1–2 years after the first Pittsburgh visit. A significantly higher proportion of patients in the rapid STPR group developed new SRC (15%) compared with the intermediate (6%) or slow group (6%) during the first year following the first Pittsburgh visit (p<0.0006). The same pattern held true for cardiac (p=0.03) and gastrointestinal disease (p<0.0001). These results are depicted in figure 2. A similar pattern was seen during the 2 years of follow-up after the first Pittsburgh visit for SRC (p=0.0005), cardiac (p=0.05) and gastrointestinal involvement (p<0.0001). There was no significant difference in the proportion of patients developing pulmonary involvement by STPR group at the end of years 1 or 2 after the first visit.
New internal organ involvement developing within 1 year of follow-up from the first Pittsburgh visit by skin thickness progression rate group (%). GI, gastrointestinal.
Multivariable regression analysis was then performed to evaluate for predictors of renal crisis, cardiac, pulmonary and gastrointestinal involvement at 1 and 2 years from the first Pittsburgh visit. The results were strikingly similar for each of the individual organ systems at 1 and 2 years (table 3). Variables examined were those easily ascertained on the first visit and included age, race, gender, autoantibody, STPR group, skin score, tendon friction rubs and the presence of other organ involvement. Rapid STPR is an independent predictor of the development of renal crisis, with a two and one-half increased odds of developing SRC in this group (OR 2.48; 95% CI 1.24 to 4.94; p=0.01) at 1 year after the first visit. At 2 years, rapid STPR confers a twofold increased risk of renal crisis (OR 2.05; 95% CI 1.10 to 3.85; p=0.02). In multivariate analysis, rapid STPR was not a significant predictor of cardiac, pulmonary or gastrointestinal involvement at either 1 or 2 years after the first visit. Decade of diagnosis and referral area (residence greater or less than 100 miles from Pittsburgh) did not affect the above model. Sensitivity analysis removing the highest STPR individuals did not change the results.
Multivariate analysis of first visit variablespredictive of SCR at 1 and 2 years of follow-up
Severity of internal organ disease
A significantly greater proportion of patients with rapid SPTR developed severe or end-stage renal disease, as assessed by the revised Medsger severity scale.2 Overall, 19% of patients with a rapid STPR developed severe renal disease at 2 years from the first visit, compared with 11% in the slow and 10% in the intermediate STPR group (p=0.006). A greater proportion of patients in the rapid STPR also developed severe cardiac disease (3%) compared with the slow (1%) and intermediate (0.5%) groups at 1 and 2 years (p=0.03). There was no greater risk of developing severe pulmonary or gastrointestinal disease for the rapid STPR group.
Discussion
The STPR is an easily performed and calculated measure designed to be used by a physician (rheumatologist) at the first visit of a SSc patient with diffuse skin thickening. We introduced this measure in a previous publication on a series of anti-topoisomerase I antibody-positive SSc patients, and found it to be a useful concept for clinicians evaluating patients with this autoantibody.3 In the present study, we explored the relationship of a rapid STPR (>45 per year) with early internal organ involvement and mortality in a large inception cohort of diffuse cutaneous SSc patients. We found that rapid STPR was an independent predictor of early mortality and the development of SRC.
Almost all recent studies of mortality in SSc have concluded that patients with diffuse skin involvement are at an increased risk of death compared with those with limited skin disease. Only a few investigators have looked at the relationship of skin thickness to mortality within the population of diffuse disease patients alone.4,–,6 Clements et al4 found that a mRSS greater than 20 predicted mortality at 4 years. Steen and Medsger6 reported that severe skin thickening with internal organ involvement was associated with poor outcome, while severe skin disease alone had a better outcome. Shand et al5 created a latent linear trajectory model of skin, using serial skin thickness scores to define three groups of patients: a high baseline skin score with no improvement group; a high baseline skin score with subsequent improvement and a low baseline skin score with later improvement. Patients with a high skin thickness score who failed to improve had a higher mortality than those with either a high skin score who demonstrated skin improvement or a lower skin score with improvement.
In our analysis, as in Shand et al,5 the skin score alone at the first visit did not affect mortality. We also examined a skin score greater than 20 at the first visit, which did not affect survival. Rather, in survival analysis we identified the group with rapidly progressive skin during their early disease as having the highest mortality. The advantage of our method is that those at highest risk can be quickly and easily identified at the first visit, rather than requiring one or more follow-up visits to establish their skin trajectory.
Steen and Medsger6 examined severe internal organ complications in SSc patients with diffuse skin thickening. For renal, pulmonary and cardiac complications, the first 3 years from disease onset was the time period in which the highest proportion of new organ systems became affected. Extending from this observation, it is our opinion that predictors of early internal organ involvement in SSc with diffuse cutaneous disease will have the greatest impact on clinical care and clinical trial design. This concept has not been explored adequately in the medical literature. Shands et al5 did examine the relationship of skin trajectory to internal organ involvement in their analysis. No difference in the frequency of individual internal organ involvement (lung, heart, kidney or skeletal muscle) was noted between the three skin trajectory groups. There was a difference between groups on the number of internal organs affected, but those with a high baseline skin score that did not improve had the lowest number, suggesting the relationship between mortality, skin thickness and internal organ involvement is complex. Similar to Shand et al,5 we found no relationship of total skin thickness score or progression to pulmonary or cardiac involvement. Neither the skin score itself, nor a skin score greater than 20 was associated with renal crisis. However, we detected a strong, independent association between rapid STPR and the development of renal crisis. The findings presented in this study are similar to those we reported in anti-topoisomerase I antibody-positive SSc patients.3 In that study, diffuse SSc patients with an intermediate and rapid STPR at the first visit were at significantly higher risk of having or developing both renal and cardiac involvement.
In the medical literature, numerous publications have concluded that many variables detected at the first evaluation of SSc patients are associated with reduced survival during long-term follow-up (5–20 years). These variables include male sex, older age, diffuse skin thickening, involvement of the heart, lung and kidney and total skin thickness score.7,–,26 We chose to focus on 2-year survival in this study so as to help us understand the natural history of early diffuse SSc before the time recommended for inclusion in clinical trials.27 In this inception cohort, only some of the previously reported significant variables (age >55 years, cardiac involvement, rapid STPR, male gender) were independent predictors of mortality. A recent study by Tyndall et al28 assessed short-term survival (median duration 0.9 years) in a multinational European SSc cohort of patients with longer disease duration and found that the presence of proteinuria, dyspnoea, pulmonary hypertension, abnormal pulmonary function tests, age at Raynaud onset and skin score predicted survival. In our analysis, rapid STPR outperformed the mRSS at the first visit in predicting both mortality and the development of renal crisis. Interestingly, although RNA polymerase III antibodies are associated with the development of SRC, anti-RNA polymerase III antibodies were modestly protective of mortality in our analysis. This may reflect the fact that although this antibody is associated with renal crisis, overall a low percentage of patients have this complication and there is available moderately effective treatment (ACE inhibitors) to prevent deaths. In addition, the lack of severity of non-renal organ involvement in anti-RNA polymerase III antibody-positive patients may favour improved survival compared with other autoantibody groups.
In our previous study using STPR, we focused on anti-topoisomerase I antibody.3 Those data showed similar results, that is, that rapid and intermediate STPR were associated with reduced survival and disproportionately frequent early renal crisis. In the case of anti-topoisomerase I, early cardiac involvement was also detected significantly more frequently in the rapid and intermediate STPR groups.
A question raised by these data is whether the STPR itself should serve as a stratification variable in the design of clinical trials in early diffuse scleroderma, especially when new internal organ involvement and early mortality are primary or secondary outcome measures.
The strengths of this study include the prospective nature of data collection and the large sample size. The primary limitation is that it was completed in a single centre tertiary care referral clinic, which may attract sicker patients with an increased number of internal organs affected. We attempted to control for this potential bias by analysing separately those patients residing within 100 miles of our centre separately and those referred from a greater distance, with similar results. Another limitation to the generalisability of our results is that ours is a predominantly Caucasian SSc population.
In diffuse cutaneous scleroderma organ involvement often occurs very early in the disease. It is in the first several years that we have the greatest opportunity to impact disease progression. Interventional studies should focus on this time period. Currently, we have limited information to identify those patients with diffuse disease who are at highest risk of early organ impairment and death. The STPR is an easily calculated bedside measure that can be performed at the time of the initial evaluation, and that serves to identify independently those diffuse SSc patients at increased risk of the development of SRC and early mortality.
Acknowledgments
The authors would like to thank Molly Vogt, PhD, for her contributions in reviewing the manuscript.
References
Footnotes
-
Funding RTD is supported by NIH 5K23AR057485.
-
Competing interests None.
-
Patient consent Obtained.
-
Ethics approval This study was approved by the Institutional Review Board at the University of Pittsburgh.
-
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Miscellaneous