Article Text
Abstract
Understanding of the natural history and basic biology of hepatitis B virus (HBV) has increased greatly in recent years. In view of this, the following are reviewed here: (a) recent advances in HBV biology pertinent to the rheumatic disease population; (b) the risks of HBV reactivation in patients with rheumatic disease undergoing immunosuppression; and (c) potential strategies to manage these risks.
- ALT, alanine aminotransferase
- anti-HBc, antibody to hepatitis B core antigen
- anti-HBs, antibody to hepatitis B surface antigen
- HBcAg, hepatitis B core antigen
- HBeAg, hepatitis B e antigen
- HBsAg, hepatitis B surface antigen
- HHBV, hepatitis B virus
- HCC, hepatocellular carcinoma
- IFN, interferon
- TNF, tumour necrosis factor
- hepatitis B virus
- biologic therapy
- immunosuppression
Statistics from Altmetric.com
- ALT, alanine aminotransferase
- anti-HBc, antibody to hepatitis B core antigen
- anti-HBs, antibody to hepatitis B surface antigen
- HBcAg, hepatitis B core antigen
- HBeAg, hepatitis B e antigen
- HBsAg, hepatitis B surface antigen
- HHBV, hepatitis B virus
- HCC, hepatocellular carcinoma
- IFN, interferon
- TNF, tumour necrosis factor
Hepatitis B virus (HBV) infection is by far the most common chronic viral infection affecting the liver in the world, with over 400 million subjects infected, and it is the leading cause of cirrhosis and hepatocellular carcinoma.1 Reactivation of HBV replication in patients undergoing immunosuppressive therapy is a well recognised and frequently reported complication of considerable clinical importance.2,3 Not surprisingly, most of these reports have come from the fields of oncology and transplantation, but there have been a growing number of cases reported in patients with rheumatic disease undergoing immunosuppressive therapy as well.4 Recent reports of HBV reactivation leading to serious complications have been described in patients with rheumatic disease undergoing treatment with biological agents, including tumour necrosis factor (TNF) inhibitors5–7 and anti-B cell therapy.8,9,10 These reports further emphasise the growing scope and complexity of this problem.
Over the past decade remarkable strides have been made in furthering our understanding of the natural history and basic biology of HBV and strategies have been proposed to assess and manage risks of reactivation in oncology and transplant recipients.11 In view of these events it would appear timely to review the following: (a) recent advances in HBV biology pertinent to the rheumatic disease population; (b) the risks of HBV reactivation in patients with rheumatic disease undergoing immunosuppression; and (c) potential strategies to manage these risks.
HEPATITIS B VIRUS INFECTION: NATURAL HISTORY AND PATHOGENESIS
Natural history
HBV, an enveloped double stranded DNA virus that belongs to the Hepadnaviridae family of viruses, infects predominantly hepatocytes, although detection of viral DNA in other sites such as mononuclear cells, pancreas, and kidneys has been documented.12 The HBV virion is a 42 nm circulating double shelled particle that is composed of an outer glycoprotein envelope containing the hepatitis B surface proteins (HBsAg), and an inner nucleocapsid or core (HBcAg) that encloses the viral DNA (approximately 3.2 kb in size) and the HBV DNA polymerase.
After entry to the circulation, HBV replicates in hepatocytes and encodes for a number of viral proteins, including the surface, core, polymerase, and X protein. HBeAg is a soluble circulating protein that is derived from the core gene after intracellular modification and is presumed to have a significant role in the establishment of persistent infection. So far, seven genotypes of HBV, designated A to G, have been identified.
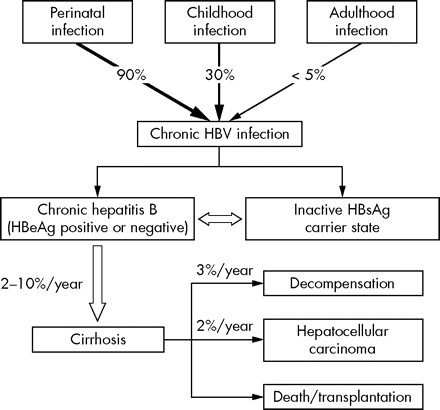
The natural course of HBV infection is influenced by a number of factors, among which the age of the initial encounter with the virus appears to be the most important (fig 1).12,13 The vast majority of chronic infection is due to vertical or perinatal transmission from HBV infected mothers. Person to person transmission during the first 5 years of life (mainly through intrafamilial spread) is another significant mode of transmission leading to chronic infection. Adulthood infection mainly occurs through injection drug use and high risk sexual behaviour and is rarely associated with chronicity (<5%). In one third of cases, acute icteric hepatitis develops that rarely can follow a fulminant course (0.5–1%).12,13
Natural course of HBV infection. Modified from reference 13.
“The natural course of HBV infection is influenced by the age of the person infected”
Acute hepatitis B is diagnosed by the presence of increased aminotransferases (alanine aminotransferase (ALT) and aspartate aminotransferase), HBsAg, and high titres of IgM antibodies against the core antigen (IgM anti-HBc).12,13 Early in the course of acute infection, HBeAg is also detectable. After the resolution of acute HBV infection (within 4–6 months), HBsAg and HBeAg disappear from the circulation and antibodies against HBsAg (anti-HBs, indicative of protective immunity) and HBeAg (anti-HBe) are detected in the serum of infected subjects (resolved HBV infection).12,13
Chronic HBV infection is defined by the persistence of the virus in the circulation for more than 6 months (HBsAg positive).13 A number of epidemiological and clinical observation studies over the past 20 years have indicated that chronic HBV infection follows a dynamic course, running through successive phases.12–14
The first or immunotolerant phase is characterised by the presence of HBeAg, high serum HBV DNA levels, and normal or near normal aminotransferase levels. For infections acquired perinatally this period lasts for decades until adulthood, whereas for infections acquired in early childhood this period ends earlier during adolescence.
During the second or immune clearance (immunoactive) phase, immunity against the replicating virus develops, leading to liver inflammation, an increase of aminotransferases and decrease of HBV DNA levels. This period usually leads to HBeAg clearance and development of anti-HBe antibodies (HBeAg seroconversion). Factors that have been associated with a high rate of HBeAg clearance include older age, female sex, increased ALT levels, and possibly, HBV genotype B. The rate of HBeAg loss has been estimated as about 50% at 5 years and 70% at 10 years, respectively. Patients who are unable to clear HBeAg and achieve HBeAg seroconversion despite active immune response develop chronic liver necroinflammation—that is, HBeAg positive chronic hepatitis B (see table 1).
Definitions and terms used in hepatitis B virus infection
HBeAg seroconversion highlights the transition to the third or low/non-replicative phase of chronic HBV infection. This phase is characterised by normal or near normal ALT levels, absent or barely detectable serum HBV DNA levels (<105 copies/ml), and the absence of significant liver necroinflammation and fibrosis. This phase is also termed inactive HBsAg carrier state.12–14 During this period, a proportion of patients can spontaneously clear the virus at a rate ranging from 0.05% to 2% a year. This occurs more often in patients who acquire infection later in life, women, and older carriers. The incidence of cirrhosis and hepatocellular carcinoma (HCC) is particularly low in this group of patients, who represent the majority of patients with chronic HBV infection in the daily clinical practice world wide.
In 20–30% of chronic HBsAg carriers, immediately after or, more commonly, years after HBeAg seroconversion, viral replication recurs, leading to host immune response and chronic hepatitis. This form of hepatitis is called HBeAg negative chronic hepatitis B.14 Analysis of the replicating HBV strains has shown that most of them possess certain mutations that abolish or significantly reduce the rate of HBeAg synthesis and secretion. A number of animal and human studies have shown that these strains emerge and predominate under the host immune pressure during the HBeAg seroconversion period.14
Chronic hepatitis B (either HBeAg positive or negative) is defined by raised ALT and HBV DNA (>105 copies/ml) levels and liver necroinflammation (documented by liver biopsy, see table 1).13 Regardless of the HBeAg status, it is associated with a number of complications, including cirrhosis, hepatic decompensation (ascites, variceal bleeding, jaundice), HCC, and death (see fig 1).13 The rate of progression to cirrhosis varies between 2% and 10% a year, while the annual rate of hepatic decompensation is about 3% (fig 1). Development of HCC occurs at an annual rate of 0.2–0.6% in non-cirrhotic patients, but this rate increases to 2% a year in cirrhotic patients. The mortality of chronic hepatitis B reaches 20% in cirrhotic patients and 70–85% in patients with hepatic decompensation at 5 years, respectively.
In recent years, patients with occult HBV infection who are persistently HBsAg negative, but with evidence of HBV DNA in the serum and/or liver tissue, have been increasingly reported.15,16 These patients belong to two major groups: the first group includes patients with a prior history of HBV infection (recovered) identified by the concomitant presence of anti-HBs and the second group, patients with chronic HBV infection. Chronically infected patients either possess HBsAg escape mutants that are not recognised by the commercially available HBsAg assays or have very low levels of viraemia with undetectable HBsAg. Some of these patients with chronic HBV infection have anti-HBc as the sole marker of HBV infection (anti-HBc only) or can be completely negative for any serological marker of HBV infection (except HBV DNA).
Pathogenesis of HBV reactivation
HBV-induced liver inflammation is predominantly immune mediated.17,18 The basic hypothesis is that HBV replication and expression of viral epitopes in infected hepatocytes is followed by a variable host immune response, leading to acute or chronic liver necroinflammation. The cells that are actively involved in this process are the cells of the adaptive immune response, predominantly CD8+ T lymphocytes, which recognise specific viral antigenic epitopes on the surface of infected hepatocytes.17,18 The role of CD4+ T cells is also crucial in this process through the provision of the required help for the production of specific antibodies against the HBV proteins (anti-HBc, anti-HBe, anti-HBs) and the local/systemic production of cytokines with antiviral activity such as interferon (IFN) γ and TNFα. Non-cytolytic mechanisms of hepatocyte viral clearance have been shown to operate in animal models of acute HBV infection through the localised action of cytokines.18,19 IFNγ is the major cytokine involved in this process, produced mainly by CD4 and CD8 T cells, macrophages, and NK T cells (a cell population particularly enriched in the liver microenvironment).
Longitudinal studies in patients with acute or chronic hepatitis B have shown that increases in serum HBV DNA are followed by a peak in ALT levels, indicating active immune response and hepatocyte lysis.2,14 This is particularly the case in patients with HBeAg negative chronic hepatitis B, where spontaneous intermittent rises in ALT levels preceded by increases in serum HBV DNA are common, leading to chronic necroinflammation and fibrosis.14
“Immunosuppressive drugs may precipitate flares of chronic HBV infection”
Among the factors that have been shown to precipitate acute flares of chronic HBV infection is the administration of immunosuppressive drugs. The bulk of available data comes from patients who have received immunosuppression for defined periods of time for haematological or oncological diseases and as long term prophylaxis after bone marrow or solid organ transplantation.2,3,20 In vitro and in vivo studies clearly indicated that immunosuppression leads to increased HBV replication, assessed by different methods (serum HBV DNA, HbsAg, and HBV DNA polymerase titres). This enhanced replication is attributed to two mechanisms. Firstly, in vitro studies have demonstrated a direct stimulatory effect of these agents on HBV replication.2 This is particularly the case for corticosteroids, because a corticosteroid responsive element is present in HBV DNA and is responsible for increased HBV DNA transcriptional activity and viraemia in patients receiving corticosteroids.21 In a recent carefully designed prospective study, increases in HBV DNA titres were noted in half of the patients within 2 weeks of the start of chemotherapy, before the development of neutropenia, indicating a potential direct stimulatory effect on HBV DNA transcription.22 Further studies are needed to confirm these preliminary findings. Secondly, an indirect immunosuppressive effect on the host immune response can be responsible for the enhanced HBV viraemia. Unopposed viral replication, followed by an exaggerated host immune response after withdrawal of immunosuppression, is the presumed pathogenetic mechanism of liver injury induced by immunosuppression.2
The rate of HBV reactivation in HBsAg positive patients receiving chemotherapy for haematological diseases or solid tumours ranges between 14% and 72%.3 These HBV exacerbations can occur either during the first cycles of chemotherapy (range 1–7 cycles) or after chemotherapy has been completed.3,20 As is the case with chronic hepatitis B, a rise in HBV DNA precedes increases in ALT by 7–10 days, strongly suggesting an immune mediated mechanism of liver injury.22–24 Similar observations have been made in patients receiving autologous haematopoietic stem cell transplantation, where immune reconstitution coincides with the exacerbation of hepatitis B (about 4 months after transplantation).25 In most cases (∼85%), viral reactivation is followed by development of clinical hepatitis.22,23 Clinically these exacerbations can be quite severe, leading to fulminant hepatitis and even death.
Despite these findings, limited information is available on the effect of the different immunosuppressive regimens given for longer periods of time in lower doses in patients with chronic HBV infection. Data from the long term administration of immunosuppressive drugs in HBsAg positive renal transplant recipients have shown a high incidence of hepatitis and liver related mortality (10–30%).20 There is a paucity of information for other diseases where long term administration of immunosuppression is required—for example, patients with inflammatory bowel diseases, severe asthma, etc.26 The data on patients with rheumatic diseases will be analysed below.
Reactivation of HBV replication or clinical hepatitis, or both, has been also demonstrated in patients with occult HBV infection after the administration of immunosuppressive drugs.8 Although the real magnitude of the problem has not been adequately estimated, a number of clinical observations indicate that is probably not large. In HBsAg negative patients with serological markers of HBV infection (anti-HBc or anti-HBs) receiving chemotherapy for haematological diseases fewer than 5% developed HBV reactivation,27 while in a recent study of HBsAg (−)/anti-HBc (+) renal transplant recipients the respective incidence was <1%.28
HBV reactivation in patients with rheumatic disease
Table 2 summarises reports of patients with rheumatic disease and HBV reactivation associated with non-biological immunosuppressive therapies.29–34
Published reports of HBV reactivation in patients with rheumatic disease treated with non-biological immunosuppressive drugs*
As can be seen, in five of the seven reports HBV reactivation was seen briefly (15–60 days) after decrease or interruption of the immunosuppressive regimen, while in two reports reactivation was noted during chronic therapy. All patients were HBsAg positive and HBeAg negative. The outcome was severe with four patients dying and one requiring liver transplantation. The other two patients were helped with antiviral therapy. Table 3 describes the published experience of HBV infected patients with rheumatic disease who have been treated with anti-TNF based therapies.4–7,35
Published experience of patients with rheumatic disease and underlying HBV infection treated with biological agents
These cases are particularly intriguing because HBV reactivation has also been recently reported in several patients treated with infliximab for Crohn’s disease, including one patient who was considered an inactive HBsAg carrier before treatment.36,37 Table 3 includes three patients treated pre-emptively with lamivudine, who had no reactivation when given infliximab despite a history of reactivation with conventional immunosuppressant drugs in one.4,6,35 Another patient was successfully treated by discontinuing the immunosuppressive drugs, including infliximab, then giving lamivudine treatment.7 The final patient5 required a liver transplant and this case is the most difficult to understand from a pathogenic perspective, for despite the progression to end stage liver disease and unlike all other cases, there was no evidence of HBV in serum or liver. It is interesting to note that at the time of writing, no cases of HBV reactivation with etanercept or adalimumab had been reported. Finally, HBV reactivation should be expected in rheumatology patients treated with ever more complex immunosuppressive regimens which include biological agents. Rituximab, for example, an agent in therapeutic trials in numerous rheumatic disorders, has been repeatedly associated with HBV reactivation in oncology treatment.8,9,10 Continued vigilance and enhanced measures to screen and prevent a flare appear prudent.
CONSIDERATIONS FOR ASSESSMENT, PREVENTION, AND TREATMENT OF HBV REACTIVATION IN PATIENTS WITH RHEUMATIC DISEASE
The field of rheumatology has no consensus guidelines for screening or treatment strategies for the prevention of HBV exacerbation in patients with rheumatological disorders undergoing immunosuppressive therapy. In the absence of such guidance we present our preferred approach based on our own clinical experience and the limited published reports (fig 2). Many of these recommendations are derived from experience gathered from patients with malignant disorders undergoing chemotherapy, or from solid organ transplant recipients.
Algorithm for assessment and prevention of HBV reactivation in patients with rheumatic disease. *Please refer to high risk groups mentioned in the text. #Includes methotrexate, leflunomide, high dose glucocorticoids, anti-TNFα agents, other biological agents (rituximab).
Reactivation of HBV infection in patients undergoing immunosuppressive therapy is characterised clinically by an increase in serum HBV DNA and ALT level. In most instances the flare of HBV is asymptomatic, although icteric flares and hepatic decompensation leading to death or requiring liver transplantation have been well documented.24 Furthermore, small studies have shown that prophylactic treatment with antiviral agents can reduce the rate of reactivation and the mortality associated with flares.20,25 Given the potentially poor outcome of HBV reactivation and effectiveness of prophylaxis in patients receiving immunosuppressive drugs, it is important to identify those at risk and implement appropriate preventive strategies.
Who should be screened?
Three strategies for HBV screening deserve consideration, including (a) universal screening; (b)screening of high risk patients; and (c) screening of those being considered for high risk treatments. No studies have examined the cost effectiveness of universal screening for HBV infection in all patients in rheumatological practice in countries where the prevalence of HBV is low and universal vaccination has been successfully implemented (North America and most of Western Europe). However, universal screening for HBV is commonly practised in these countries, especially for those being assessed for organ transplantation. In the absence of data suggesting a more prevalent problem (that is, HBV reactivation), universal screening cannot be supported.
In contrast, screening for those at high risk for HBV infection will probably prove to be an effective strategy and one that is economically viable. At risk patients include people born in endemic areas, men who have sex with men, injection drug users, renal dialysis patients, HIV infected patients, and household or sexual contacts of HBV infected patients. Identification of those at-risk subjects requires careful and detailed medical history before initiation of immunosuppressive treatment.
“Screening of high risk patients is a more effective strategy than universal screening”
Lastly, HBV screening should be considered for all patients going onto immunosuppressive regimens which have the potential to induce reactivation of HBV infection. At present the only formal recommendations in rheumatology are those to screen high risk patients starting treatment with methotrexate or leflunomide.38 Given the recent reports of serious even fatal cases of HBV reactivation with the drug infliximab,6,35–37 it would appear prudent to consider screening all patients who are to be treated with a member of the TNF inhibiting class.
Finally, although an absolute minimal level of immunosuppressive/immunomodulatory activity below which HBV reactivation is no longer a risk cannot be defined at present, it appears unlikely to occur in patients given low dose glucocorticoid treatment (<7.5 mg of prednisone or equivalent a day), with or without such agents as antimalarial drugs, sulfasalazine, and gold compounds, because to date there are no such reports. Alternatively, for patients who are to receive high dose glucocorticoid treatment, with or without a cytotoxic drug (that is, an alkylator or antimetabolite) or a calcineurin antagonist, screening for HBV should be strongly considered.
A decision about screening is more difficult for all patients (not just those at high risk) who are to be treated with methotrexate or leflunomide based regimens. The data in table 2 show that reports of severe, even fatal, reactivation of HBV have been documented when these drugs are used and, although rare, suggest that serious consideration should be given to screening all patients treated with these agents. Screening of patients merely at high risk will lead to missing one in four infected patients who lack identifiable risks.
What test should be used for screening?
HBsAg testing of all at-risk subjects, should be carried out. Patients with detectable serum HBsAg should receive prophylaxis before immunosuppressive treatment, as discussed later in this manuscript. Whether a more detailed serological testing including anti-HBc and anti-HBe is needed for the screening of patients at risk remains unknown. HBV reactivation can occur in people who are HBsAg negative but anti-HBs and anti-HBc positive.11,24,27 Similarly, HBV reactivation is well documented in solid organ transplant recipients of an anti-HBc positive donor, and prophylaxis with antiviral agents (short term) has been recommended for those patients.39 Given these data, we recommend screening of patients at risk for HBV reactivation with a serological profile that would include HBsAg, anti-HBs and anti-HBc.
Who should receive prophylactic therapy?
Treatment of all HBsAg positive patients should be started with prophylactic antiviral drugs before they receive immunosuppressive therapy including corticosteroids. For HBsAg negative, anti-HBs, and/or anti-HBc positive patients, prophylactic therapy may not be necessary routinely and the decision can be made individually based on the likelihood of reactivation (type of immunosuppressive therapy, length of treatment, etc.). If no antiviral prophylactic treatment is given in this group of patients, periodic follow up of ALT and HBV DNA is recommended to identify flares at an early asymptomatic stage, at which time treatment of HBV can be started before the onset of jaundice or liver failure.
STRATEGIES FOR PROPHYLAXIS
IFNα has no role in the prophylaxis against HBV reactivation for those undergoing immunosuppressive therapeutic regimens. Similarly, the use of hepatitis B immunoglobulin (HBIG) prophylaxis should be limited to patients with an actively replicating HBV infection (HBsAg positive) undergoing liver transplantation and has no role in prophylaxis in patients undergoing immunosuppressive therapy, such as patients with cancer or patients with rheumatological disorders.
Although no randomised clinical trials have been completed, most reports to date have demonstrated the benefit of lamivudine given prophylactically (100 mg daily) to patients with cancer undergoing chemotherapy.23,40,41 It has been suggested that lamivudine should be given throughout the course of treatment and extended for 6 months after completion of the chemotherapy regimen because HBV flares may occur days or weeks after chemotherapy has stopped.27,42 Short term lamivudine is safe and, usually free of toxicity with a risk-benefit ratio that favours prophylaxis. A randomised study showed that lamivudine given 1 week before administration of chemotherapy was better than deferred lamivudine treatment given at the time of viral reactivation.23
In a small number of patients with rheumatic disease who had reactivation of HBV during an immunosuppressive regimen, lamivudine was successfully employed to suppress HBV replication, allowing successful reinstitution of treatment.4,35 The benefit versus risk of prophylactic antiviral therapy to prevent HBV flares is less certain in those requiring an extended course of immunosuppressive therapy. A longer course of lamivudine prophylaxis may potentially be associated with emergence of lamivudine resistant HBV strains (through mutation in the YMDD motif of the HBV polymerase). This is of particular concern in rheumatic disorders where immunosuppressive regimens including corticosteroids may be given over an extended period of time or sometimes for life. The annual risk of developing the YMDD mutation increases with the duration of treatment in immunocompetent patients with chronic hepatitis B (HBeAg positive or negative). The rate of virological breakthrough due to YMDD mutant strains has been estimated as 15–30% at year 1, 40% at year 2, 50% at year 3, and 60% at year 4 of treatment.43,44 The clinical course of patients with lamivudine resistant HBV strains is variable and may rarely lead to hepatic decompensation.45 This is particularly the case for patients with advanced fibrosis or cirrhosis.43 However, most patients with the YMDD mutation are asymptomatic or may have mild exacerbation of hepatitis.
Limited information is available on the rate of lamivudine resistance in immunosuppressed subjects. Long term immunosuppression in kidney transplant recipients has been associated with a number of mutations in different areas of the HBV genome,46 but whether the rate of YMDD mutations is higher in these patients is unclear. A recent meta-analysis of lamivudine treatment in renal transplant recipients with chronic HBV infection showed a rate of lamivudine resistance that ranged between 10% and 42%.47 In a study by Chan et al, where long term follow up was available (approximately 2 ½ years), the rate of YMDD resistance was 7% and 37% after 1 and 2 years of treatment, respectively.48 These rates are similar to the rates seen in immunocompetent HBV patients. Nevertheless, increased vigilance with frequent monitoring of HBV DNA and ALT levels is imperative in these patients, especially when advanced liver fibrosis is present.
Adefovir dipivoxil, an alternative antiviral drug, has not been studied in the setting of prophylaxis during immunosuppressive therapy. Adefovir dipivoxil has been shown to be effective in the treatment of patients with chronic hepatitis B49,50 and in those developing YMDD mutation during lamivudine treatment.51–53 The recommended dose of adefovir dipivoxil in adults is 10 mg daily given orally. So far, long term administration of adefovir dipivoxil has been associated with a much lower rate of drug resistance than lamivudine (∼15% after 4 years of treatment).54 Combination therapy with lamivudine and adefovir has been proposed for patients with advanced liver disease in order to avoid severe decompensation in cases where resistance to lamivudine monotherapy develops. Whether such an approach should be implemented in patients with HBV cirrhosis who will receive immunosuppressive therapy is unclear. Other antiviral agents are currently under development and may potentially provide alternative treatments to prevent HBV reactivation.44