Article Text
Abstract
OBJECTIVES To investigate the progression of joint damage in early rheumatoid arthritis (RA) using magnetic resonance imaging (MRI) of the wrist and determine whether this technique can be used to predict prognosis.
METHODS An inception cohort of 42 early patients has been followed up prospectively for one year. Gadolinium enhanced MRI scans of the dominant wrist were obtained at baseline and one year and scored for synovitis, tendonitis, bone marrow oedema, and erosions. Plain radiographs were performed concurrently and scored for erosions. Patients were assessed clinically for disease activity and HLA-DRB1 genotyping was performed.
RESULTS At one year, MRI erosions were found in 74% of patients (31 of 42) compared with 45% at baseline. Twelve patients (28.6%) had radiographic erosions at one year. The total MRI score and MRI erosion score increased significantly from baseline to one year despite falls in clinical measures of inflammation including erythrocyte sedimentation rate (ESR), C reactive protein (CRP), and swollen joint count (p < 0.01 for all). Baseline findings that predicted carpal MRI erosions at one year included a total MRI score of 6 or greater (sensitivity: 93.3%, specificity 81.8%, positive predictive value 93.3%, p = 0.000007), MRI bone oedema (OR = 6.47, p < 0.001), MRI synovitis (OR = 2.14, p = 0.003), and pain score (p = 0.01). Radiological erosions at one year were predicted by a total MRI score at baseline of greater than 13 (OR = 12.4, p = 0.002), the presence of MRI erosions (OR = 11.6, p = 0.005), and the ESR (p = 0.02). If MRI erosions were absent at baseline and the total MRI score was low, radiological erosions were highly unlikely to develop by one year (negative predictive value 0.91 and 0.92 respectively). No association was found between the shared epitope and erosions on MRI (p = 0.4) or radiography (p = 1.0) at one year.
CONCLUSIONS MRI scans of the dominant wrist are useful in predicting MRI and radiological erosions in early RA and may indicate the patients that should be managed aggressively. Discordance has been demonstrated between clinical improvement and progression of MRI erosion scores.
- magnetic resonance imaging
- carpus
- rheumatoid arthritis
- prognosis
Statistics from Altmetric.com
Early rheumatoid arthritis (RA) is characterised by inflammation of peripheral joints that proceeds to chronic synovitis and erosive bony change after a period of months to years.1 Early treatment with disease modifying anti-rheumatic drugs (DMARDs) has been advocated to slow disease progression and improve long term prognosis.2 ,3 However, many patients with peripheral inflammatory symmetrical arthritis (PISA)4 do not go on to develop erosive RA and the condition resolves spontaneously in up to 50%. Clearly, treatment of these patients with potentially toxic drugs such as methotrexate would be inappropriate. Historically, many clinicians preferred to wait for the development of radiological erosions before confirming a diagnosis of RA and embarking on treatment with the more potent DMARDs. Unfortunately, erosions may not be revealed by radiography until disease has been established for 12 months or more5resulting in an unacceptable delay for those with aggressive disease. Magnetic resonance imaging (MRI) of the wrist has been shown to reveal erosions more frequently than radiography in several cross sectional studies of early RA6-8 and may be useful in identifying at risk RA patients at first presentation. However, the progression of joint damage using MRI has only been examined longitudinally in a small number of patients9 ,10 and the sensitivity and specificity of this technique for erosive change has not been evaluated.
We have devised a reliable scoring system for MRI of the wrist in RA and have applied this to a cohort of 42 patients with very early disease (mean symptom duration at baseline of four months).11 Baseline MRI scans revealed erosions of the dominant wrist in 45% of patients while only 15% had erosions on plain radiography, suggesting that MRI may be a useful tool in early RA for defining joint damage and identifying those with aggressive disease. We now report the analysis of follow up MRI scans and radiographs at one year for this group. Results have been compared with baseline to look for early MRI changes that identify those who go on to develop erosive disease and who may have a less favourable prognosis.
Methods
PATIENT POPULATION AND CLINICAL ASSESSMENTS
An inception cohort of 42 patients with early RA has been studied since symptom onset. Details of recruitment, baseline patient characteristics, and clinical assessments have been described.11 To briefly summarise, all patients fulfilled 1987 ARA criteria for RA12 and had had symptoms for six months or less (median four months) at entry to the study. All patients were assessed clinically at baseline by a rheumatologist (FM) to determine disease activity. Plain radiographs of hands and feet were obtained as well as MRI scans of the dominant wrist.
At the one year follow up visit, these investigations were repeated and table 1 summarises characteristics of the group at both time points.
Patient characteristics at baseline and one year (n=42)
MRI SCANS
An MRI scan of the dominant wrist was obtained using a 1.5 Tesla MR scanner (GE Signa Horizon) with a dedicated wrist coil (Medical Devices). The field of view was 8 cm and included the distal radioulnar, radiocarpal, and mid-carpal joints as well as the metacarpal bases. The small field of view was chosen to optimise resolution and did not include metacarpophalangeal (MCP) joints. Coronal and axial T1 sequences were performed, followed by axial fat suppressed fast spin echo T2, then coronal fat suppressed T1 sequences after injection of gadolinium (Nicomed Omniscan).
MRI SCORING SYSTEM
The system used to score MRI scans has been described elsewhere.11 Briefly, erosions and bone marrow oedema were scored at each of the 10 carpal bones and the five metacarpal bases. Synovitis was scored at seven individual sites on the basis of synovial thickening and enhancement after gadolinium injection. Tendonitis was evaluated on axial scans and scored at each of nine tendon groups. A total MRI score for the carpus was derived from the sum of scores for erosions, bone marrow oedema, synovitis, and tendonitis.
MRI scans were scored independently (and without reference to radiographs) by two musculoskeletal radiologists who were blinded to clinical and genetic data. One year scans were read and scored approximately one year after baseline scans and so the sequence of scans was known. However, the radiologists did not have access to baseline scans for comparison, when one year scans were scored. Interobserver reliability of the scoring system at baseline was high (total MRI score, r = 0.81) as was intraobserver reliability (r = 0.94 and 0.81 for observers 1 and 2).13 Further validation was performed at one year as the range of scores was greater. Measures of interobserver reliability were as follows: total MRI score; r = 0.87, (CI: 0.77, 0.93), synovitis score; r = 0.90 (0.82, 0.95), erosion score;r = 0.75 (0.53, 0.87), tendonitis score;r = 0.73 (0.54, 0.84). Where observers differed as to the presence or absence of erosions, scans were reviewed and a consensus opinion obtained.
RADIOLOGY
Radiographs of the hands and feet were taken using standard views. For each patient, films of the dominant carpus were scored for erosions within the same field of view as shown on MRI scans, by two musculoskeletal radiologists using criteria defined by Sharp.14 Radiologists scored the radiographs separately (blinded to MRI scores) but where there was dissent regarding the presence or absence of erosions, a consensus opinion was obtained. Scoring of radiographs of both hands and feet for erosions was also performed separately by both radiologists. Details of interobserver and intraobserver reliability for radiographic scoring at baseline have been published.11
GENETIC STUDIES
Methodology used for HLA-DRB1 typing has already been described.11 Briefly, DNA was extracted from anticoagulated blood obtained from each patient at recruitment. Low resolution typing was performed using sequence specific primer polymerase chain reaction (SSP-PCR) with a standardised panel of 24 oligonucleotide primer pairs.15 In subjects with alleles of the DRB1*04 or 01 groups, the sequence of the subtype determining region of exon 2 of the DRB1 gene(s) was obtained by direct sequencing of PCR products.16
A comparison of high resolution HLA-DRB1 typing methods in this group has been reported elsewhere.17
STATISTICS
Intraclass correlation coefficients were calculated to investigate the inter-rater reliability of the MRI scores.13 Each person was rated once by each observer and the observers were considered to be a random sample of all possible observers so that the results could be generalised. A Wilcoxon signed ranks test was used to test for significant changes between baseline and one year for clinical variables.18 Logistic regression was used to investigate whether baseline clinical measurements were able to predict the presence of erosions at one year. Best subsets selection and the coefficient of determination (a generalisedr 2) were used to determine the predictive models that best explained most variation.19χ2 Tests, Fisher’s exact tests and logistic regression (exact where appropriate) were used to test for associations between erosions at one year and other measures. Receiver operating characteristic (ROC) curves were constructed by varying the cut off point in the total MRI score at baseline for prediction of radiological and MRI erosions at one year.20 The cut off point that maximised the sum of the specificity and sensitivity for this test was calculated.
For statistical analysis of the relation between MRI bone oedema at baseline and MRI erosion at one year, the following were used; baseline data for presence of bone oedema (as scored by one or both observers) versus one year data for presence of MRI erosions (consensus opinion). Only those baseline sites with oedema but no erosions were chosen. Two analyses were performed. Firstly, each finding of bone oedema at baseline was assumed to be an independent occurrence and sites for all patients were combined. A χ2 test was used to investigate the relation between erosions and oedema.
Secondly, a repeated measures analysis21 was used, selecting the 10 most common sites for bone oedema at baseline, to account for the correlation between measurements in the same patient (see Results section). The same procedures were used to investigate whether synovitis at baseline was associated with development of erosions at adjacent carpal bones at one year. Only the 10 most common sites for synovitis were included in the analysis and only those sites at which there was synovitis but no erosions at baseline.
Results
CLINICAL MEASURES OF DISEASE ACTIVITY AT ONE YEAR COMPARED WITH BASELINE
Table 1 shows the clinical data at baseline and one year for the 42 study patients. A marked reduction in joint inflammation was apparent at one year (mean duration of symptoms, 16 months). Improvement was seen in subjective clinical measures such as pain score (p= 0.03) as well as objective parameters including swollen joint count, ESR, and CRP (p< 0.001 for all). The HAQ score also fell from a baseline median value of 0.6 to 0.1 at one year (p=0.04) reflecting an improvement in function that paralleled a reduction in clinical evidence of inflammation. Changes in medication were also observed with a reduction in overall NSAID use (90% of the group at baseline to 60% at one year) and an increase in patients taking DMARDs (50% at baseline to 71% at one year); predominantly sulphasalazine and methotrexate (table 1). Patients taking low dose corticosteroid (1–10 mg prednisone) increased from 12% at baseline to 24% at one year.
MRI SCORES AT ONE YEAR COMPARED WITH BASELINE
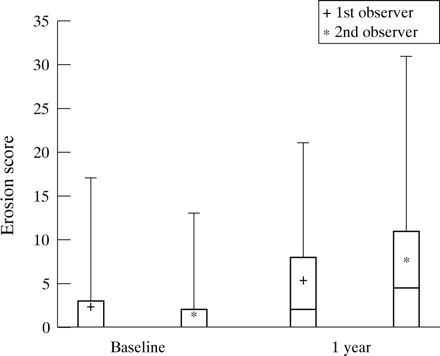
Despite the apparent clinical improvement, total MRI scores for both observers rose significantly from baseline to one year (p = 0.02). Erosion scores also increased significantly (p < 0.001) and are depicted in figure 1 In contrast, MRI synovitis scores did not significantly change between baseline (mean scores of 6.6 and 8.3) and one year (mean scores of 7.4 and 6.6). There was also no change in mean bone oedema or tendonitis scores. Thus the increase in the total MRI scores from baseline to one year was because of a rise in erosion scores.
MRI erosion score at baseline and one year for observer 1 and observer 2. Box plots show the mean (x), median (-), interquartile range (box) and spread of the data.
PROPORTION OF PATIENTS WITH MRI EROSIONS INCREASES FROM BASELINE TO ONE YEAR
At baseline, 19 of 42 patients (45%) had erosions on MRI (fig 2). At one year, all patients had follow up scans that revealed erosions in 31 of 42 (73.8%). Of those with erosions at baseline on MRI, 18 of 19 patients still had erosions at one year. One patient was scored as having one erosion at baseline but none at one year. Of those patients without erosions at baseline, 13 of 23 had developed erosions on the one year scan. Ten patients remained non-erosive at baseline and one year. Figure 3 (A–D) shows carpal MRI scans for one of the study patients illustrating progression from bone marrow oedema at baseline to multiple bony erosions at one year. Interestingly, patients taking DMARDs were more likely to have MRI erosions at one year than those not taking those drugs (p = 0.006), probably reflecting more active disease.
Progression of erosions on MRI and radiography from baseline to one year.
(A) Coronal T1 weighted MRI scan of the dominant carpus of a study patient at baseline showing bone marrow oedema of the capitate (arrow). (B) Coronal T1 scan of the same patient at one year showing progression of MRI changes with erosions of the hamate, capitate, scaphoid, lunate, triquetrum and distal radius (arrows). (C) Axial T1 scan at baseline showing synovial thickening within the distal radioulnar joint and radiocarpal joint adjacent to the palmar aspect of the distal radius (asterixes). (D) Axial T1 scan of the same patient at one year showing a large erosion that has developed within the palmar aspect of the distal radius (arrow).
MRI EROSIONS MAY PRECEDE THE DEVELOPMENT OF RADIOLOGICAL EROSIONS
Twelve of 42 patients (28.6%) had radiological erosions of the dominant carpus at one year (fig 2). Of those, 10 had MRI erosions both at baseline and one year and two had MRI erosions at one year only (table 2). The impression of the radiologists examining MRI scans was that MRI erosions usually appear several months before radiological erosions. Logistic regression found the association between MRI erosions at baseline and radiological erosions at one year to be significant (OR = 11.6, χ2 (1df) = 8.0, p = 0.005) confirming this. The sensitivity of baseline MRI erosion for prediction of radiological erosion at one year was 83% while the specificity was 70% giving a positive predictive value (ppv) of 0.58 and a negative predictive value (npv) of 0.91 for this test.
MRI erosions at baseline and one year compared with radiological erosions at one year
BASELINE TOTAL MRI SCORE PREDICTS EROSIONS AT ONE YEAR
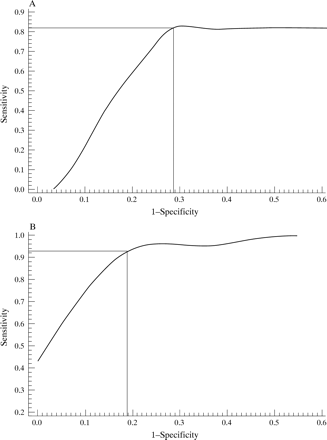
A baseline total MRI score was calculated for each patient from the mean score of both observers. ROC curves20 were constructed to explore the relation between baseline total MRI score and carpal erosions on MRI and radiography at one year (figs 4A and4B). The curve plots sensitivity versus 1−specificity and its apex marks the point at which the most favourable sensitivity and specificity is produced. For prediction of MRI erosions at one year, a cut off at 6 was optimal resulting in a significant association (p = 0.000007) (table 3), with a sensitivity of 93.3% and a specificity of 81.8%, ppv = 0.93. Thus 93% of patients with a baseline total MRI score of > or equal to 6 developed MRI erosions at one year. A higher cut off point of 13 for baseline total MRI score was predictive of radiological erosions at one year (OR = 12.38, p = 0.002). This produced a sensitivity of 82%, specificity of 73%, ppv of 0.53 and npv of 0.92. The same analysis was applied to prediction of radiological erosions at the hands or feet at one year. Sixteen patients with a baseline MRI score of 10 or greater developed erosions at one year compared with 11 with a baseline score of less than 10 but this difference did not reach significance (p = 0.3).
(A) Receiver operating characteristic (ROC) curve for the prediction of MRI erosions at one year by baseline total MRI score. The apex of the curve defines the point at which the optimum sensitivity and specificity for this test are achieved (see text). (B) ROC curve for the prediction of radiological erosions at one year by baseline total MRI.
Baseline MRI features that predict MRI erosions at one year
BONE MARROW OEDEMA PRECEDES THE DEVELOPMENT OF MRI EROSIONS
Data were analysed to determine how often bone oedema at baseline was followed by erosion at that site at one year. A total of 542 paired observations were examined and a significant association between bone oedema at baseline and erosion at one year was found. The odds ratio calculated for this association, assuming each site to be affected independently (Methods, statistics section), was 6.47 (95%CI: 3.23, 13.05), χ2 (1df) = 38.96, p < 0.0001 (table 3). Thus, those patients with bone oedema at a specific site on baseline MRI were 6.5 times more likely to develop erosions at that site at one year than those without bone oedema. A more rigorous repeated measures analysis21 was also performed (using the 10 most common sites) to account for correlation between sites in the same patient. This also found an association between bone marrow oedema at baseline and erosion at the same site at one year (p=0.04).
SYNOVITIS AT BASELINE PREDICTS MRI EROSIONS AT ONE YEAR
Results were analysed to determine whether synovitis at one of the carpal joints at baseline was likely to be followed by erosion at an adjacent bone at one year. Only bony sites where there were no baseline erosions observed were included. A repeated measures analysis (using the 10 most common sites) found that synovitis at baseline had a significant effect on the presence of erosions at one year (p = 0.0004) (table 3).
CAN BASELINE CLINICAL TESTS PREDICT MRI OR RADIOLOGICAL EROSIONS?
Logistic regression was used to determine the baseline clinical features that were predictive of MRI and radiological erosions at one year. The best single predictor of MRI erosions was the pain score (χ2 (1df) = 6.23, p = 0.01,r 2 (adj) = 0.30). When pain score, DAS, and CRP were considered together, the variance explained was increased (r 2 (adj) = 0.57). The ESR was the best single predictor of radiological erosions (χ2 (1df) = 5.72, p=0.02,r 2 (adj) = 0.22). Including the tender joint count, DAS and swollen joint count increased the variance explained (r 2 (adj) = 0.47).
PRESENCE OF THE SHARED EPITOPE DOES NOT PREDICT MRI OR RADIOLOGICAL EROSIONS
Baseline data from this cohort of patients did not show any association between MRI erosions and the presence of the shared epitope (SE).11 This question was re-examined at one year using MRI scans and radiographs of the dominant wrist. Of the 42 patients, 32 were SE positive (76%). Of these, 25 (78%) had erosions on MRI at one year, compared with 6 of 10 SE negative patients (60%). This difference was not significant (p = 0.4). Using radiographic data, 9 of 32 SE positive patients were shown to have erosions at one year (28%) compared with 3 of 10 patients (30%) of SE negative patients (p = 1.0) (table 4).
Shared epitope vs MRI and XR erosions at one year
Discussion
Carpal MRI is a more sensitive test for detecting erosions in early RA than plain radiography.6-8 We have shown 45% of our cohort of patients to have MRI erosions at baseline11and this rose to 74% at one year. This compares with radiological erosions that increased from 14% to 29% over the same period. It is important to know whether MRI erosions are early transient lesions or whether they are persistent and associated with progressive joint damage. Our results suggest that the latter is generally true. Of the 19 patients with MRI erosions at baseline, all but one still had MRI erosions at one year. Scans from the one patients who “lost” her erosions at one year were reviewed. The baseline scan had initially been scored as non-erosive by one radiologist and erosive by the other (one erosion only). In retrospect, this was felt to be a false positive because of the presence of intense carpal synovitis adjacent to an area of bony irregularity. On the one year scan, synovitis had settled and no erosion was identified.
The relation between MRI and radiological erosions is also important to define. Of those patients with MRI erosions at baseline, 58% had developed radiological erosions at one year. There seemed to be a lag phase of 6 to 12 months between the appearance of MRI and radiological erosions in individual patients. Thus, of those patients with radiological erosions at one year, 80% had had MRI erosions at baseline while in the other 20%, MRI erosions had become apparent on the one year scans. This temporal association between MRI and radiological erosions was highly statistically significant but the ppv was relatively low at 0.58. This may be an underestimate, both because of the difficulty visualising radiological erosions in the carpus because of its three dimensional structure and because in some patients, radiological erosions may take longer to develop. Further analysis of radiographs from this cohort at the two year time point is planned to clarify this. A very high npv of 0.91 was found for the association implying that in 91% of patients without MRI erosions at baseline, there were no radiological erosions at one year. This information might be useful clinically, as the finding of no erosions on baseline MRI scans could be used to classify patients as having mild disease, thereby influencing the decision to treat with potentially toxic DMARDs.
The total MRI score at baseline was found to be highly sensitive and specific for the prediction of carpal MRI and radiological erosions at one year. A baseline total MRI score of 6 or > gave a ppv of 0.93 for the development of MRI erosions at one year. A score greater than 13 was predictive of radiological erosions at one year with a ppv of 0.53. Once more, this lower predictive value for radiological erosions may be explained by their slower development as they would only be apparent at one year in those patients where joint damage was already well established at baseline (reflected by a high total MRI score). Again the npv for this association was very high at 0.92. Thus, those with a baseline MRI score of 13 or less were very unlikely to develop radiological erosions at one year and this could be used as a guide to clinical practice as described above. While the total carpal MRI score at baseline was a good indicator of long term joint damage at that site, we did not find it to be predictive of “overall” erosive disease assessed on radiographs of the hands and feet at one year. This may be because erosions at different sites progress at different rates as found by Brook et al who noted the feet to become involved much earlier than the hands.5 Again, further analysis of radiological erosions at all sites at two years may provide stronger evidence for the predictive accuracy of baseline carpal MRI scores.
We have analysed baseline MRI scans to look for early changes that may precede joint erosion at one year. Both bone marrow oedema and synovitis were significantly predictive of erosion when analysed in a site specific manner and seem to be early indicators of potentially aggressive disease. The significance of early bone marrow oedema progressing to bony erosion at one year has also probably been underestimated as we have only examined instances where oedema was present alone at baseline. In many cases, it was present together with erosion at baseline and a causative link could not be established. The design of the present study with two “snapshots” taken one year apart does not allow a clear picture to be built up of the progression of MRI changes during the first months of disease and this would require more frequent scans over shorter time intervals. Other investigators have also suggested a sequence of progression of MRI changes in early RA, from synovitis to bone oedema to loss of oedema and development of erosion.10 Moreover, Leeet al 9 have described a decrease in MRI bone oedema and synovial proliferation in RA patients in remission after treatment with DMARDs.
How does carpal MRI compare with clinical measures for prediction of disease severity? For this cohort, pain score and ESR at baseline were the best single predictors of MRI and radiological erosions respectively at one year. These clinical measures were much less strongly predictive of erosion than baseline MRI findings as described above. Other authors have looked for clinical indicators of prognosis in early RA with variable results. Van der Heidjeet al found high initial disease activity as measured by the ESR, CRP or DAS to be predictive of radiological outcome.22 Fex et al 23 also found that high baseline ESR significantly predicts an increased rate of radiological progression during the first year but, in their study, active joint counts and HVR3 status had no predictive value.
The medical literature regarding the importance of the shared epitope in disease prognosis is also divided. Nepom et al 24 have stated that HLA-DR4 genes can predict progression to erosive disease with a sensitivity and specificity of 75–80% in white populations. The findings of Goughet al 25 supported this and emphasised the predominance of the Dw4/Dw14 genotype in patients with early erosive disease. However, Eberhardt et al 26 did not find a strong association between genotype and disease progression in their group of 84 RA patients and van Jaarsveld et al 27 recently confirmed this for a Scandinavian population using genomic tissue typing. Studies in Japanese28 and Pakistani29patients have also failed to find a link between the shared epitope and radiological progression. In our comparatively small group of 42 patients, no association was observed between erosions on MRI or radiography and the presence of the shared epitope, either at baseline or one year. However, a larger number of patients would need to be studied to detect a weak association and avoid a type II error. It may also be relevant that our group was diluted by nine Polynesians in whom HLA associations with disease severity may differ from those of European extraction.30
An important observation from this study was the discordance between clinical evidence of joint inflammation and MRI findings. While clinical scores improved during the one year study period, with significant reductions in swollen and tender joint counts, HAQ scores, CRP and ESR, MRI synovitis scores did not change. This suggests that MRI may detect subclinical synovitis that could persist despite apparent clinical improvement. Other groups have reported a similar discrepancy between clinical and MRI observations including Reiseret al 31 who noted no correlation between MRI and clinical synovitis at the knee in RA patients, and Jorgensen et al who confirmed these findings at the wrist in early disease.32 Jevticet al 33 examined this question longitudinally over 24 months and found only 58% congruence between clinical and MRI disease activity in RA. We are currently analysing dynamic MRI scans from our patients taken at baseline and one year, as these should provide more accurate and objective information about the degree of synovitis than can be obtained from scoring static MRI scans.
In contrast with synovitis scores, MRI erosion scores from our patients increased inexorably over one year and were markedly discordant with the clinical improvement observed. This is consistent with observations by groups using plain radiology to assess disease progression. A striking contrast between clinical improvement and radiological deterioration during the first six years of RA was observed by Mulherinet al 34 who postulated that the pathological processes underlying joint inflammation may differ from those resulting in erosion and articular damage. Kirwan35reported similar conclusions from his study of low dose corticosteroids in early RA, where he noted an improvement in joint inflammation scores in DMARD treated placebo and prednisolone groups, but less radiological progression in the prednisolone group suggesting a specific articular protective effect from this drug. Evidence from immunohistological studies also supports the idea of separate pathological processes underlying rheumatoid joint disease, possibly mediated by separate cytokines. Anti-tumour necrosis factor α and anti-interleukin 1 agents have both been observed to have powerful anti-inflammatory effects in collagen induced arthritis but only the anti-interleukin 1 agent was successful in reducing cartilage destruction.36This suggests that agents targeting tumour necrosis factor α in early RA may not prevent joint erosion and MRI is likely to be of great importance in determining this in future clinical trials. Although many patients in our study received DMARDs, these did not seem to halt the development of erosions that actually appeared more commonly in this group. This probably simply reflects the fact that those receiving DMARDs were those with more active disease, but it does emphasise that conventional treatment has only a minor impact on the progression of erosions.37
In summary, this longitudinal, prospective study of patients with early RA has shown carpal MRI to reveal erosions earlier and in a much greater proportion of patients than plain radiography. We have observed a progression from early MRI changes of synovitis and bone oedema, to MRI erosion and finally radiological erosion, over a period of about 12 months. A high total carpal MRI score at baseline was strongly predictive of MRI erosions at one year and a low score predicted a lack of carpal erosions on radiography at one year. However, MRI of the wrist could not predict “overall” erosions at the hands and feet and no association was found between erosions and the shared epitope. Finally, an interesting disparity between clinical improvement and MRI deterioration was observed in our patients during the first year of their disease, suggesting that different pathophysiological processes could underlie synovial inflammation and articular erosion. Biological agents may allow these processes to be targeted separately and MRI scans could play a useful part in evaluating their influence on disease progression in early RA.
Acknowledgments
The authors wish to acknowledge the assistance of the following clinicians who have referred patients for this study: Dr Mike Butler, Dr David Caughey, Dr Nora Lynch, Dr Alan Doube, Dr Hamish Hart, Dr Peter Gow, Dr Raoul Stuart, Dr Terry Macedo, Dr Max Robertson, Dr Roger Reynolds, Dr Bob Grigor. We are also most grateful to the technical staff at the Auckland Radiology Group who supervised the MRI scans and to Mrs Ma Wei (technician, Department of Molecular Medicine) who performed HLADRB1*04/01 genotyping.
References
Footnotes
Funding: supported by grants from the Health Research Council of New Zealand, the Arthritis Foundation of New Zealand, the Auckland Medical Research Foundation, the Auckland Radiology Group and Sanofi-Winthrop.