Article Text
Abstract
Psoriasis is a common chronic, recurrent, immune mediated disease of the skin and joints. It can have a significant negative impact on the physical, emotional, and, psychosocial wellbeing of affected patients. Psoriasis is found worldwide but the prevalence varies among different ethnic groups. It has a strong genetic component but environmental factors such as infections can play an important role in the presentation of disease. There are several clinical cutaneous manifestations of psoriasis but most commonly the disease presents as chronic, symmetrical, erythematous, scaling papules and plaques. The epidemiology, clinical features, and impact on quality of life of psoriasis are reviewed.
- PASI, Psoriasis Area and Severity Index
- PsA, psoriatic arthritis
- PSI, Salford Psoriasis Index
- RA, rheumatoid arthritis
- clinical
- epidemiology
- psoriasis
- quality of life
Statistics from Altmetric.com
- PASI, Psoriasis Area and Severity Index
- PsA, psoriatic arthritis
- PSI, Salford Psoriasis Index
- RA, rheumatoid arthritis
This paper reviews the epidemiology and clinical features of psoriasis and its impact of patients’ quality of life.
EPIDEMIOLOGY
Although psoriasis occurs worldwide, its prevalence varies considerably. In the USA, approximately 2% of the population is affected. High rates of psoriasis have been reported in people of the Faroe islands, where one study found 2.8% of the population to be affected.1 The prevalence of psoriasis is low in certain ethnic groups such as the Japanese, and may be absent in aboriginal Australians2 and Indians from South America.3
Psoriasis can present at any age and has been reported at birth and in older people of advanced age. Accurate determination of the age of onset of psoriasis is problematic, as studies which do so typically rely on a patient’s recall of the onset of lesions or determine the onset from the physician’s diagnosis as recorded on the initial visit. Data based on patient recall can be inaccurate; determining onset based on first visit to a physician could underestimate the time of disease occurrence, as minimal disease may be present for years before a consultation is sought. A bimodal age of onset has been recognised in several large studies. The mean age of onset for the first presentation of psoriasis can range from 15 to 20 years of age, with a second peak occurring at 55–60 years.4–7
Henseler and Christophers examined a series of 2147 patients and reported two clinical presentations of psoriasis, type I and II, distinguished by a bimodal age at onset. Type 1 begins on or before age 40 years; Type II begins after the age of 40 years. Type I disease accounts for more than 75% of cases.7 Patients with early onset, or type I psoriasis, tended to have more relatives affected and more severe disease than patients who have a later onset of disease or type II psoriasis. In addition, strong associations have been reported with human leucocyte antigen (HLA)-Cw6 in patients with early onset, compared with later onset of psoriasis. The course and progress of psoriasis is unpredictable. In one study, 39% of patients reported complete remission of disease for between one and 54 years.8 Higher figures have been reported in Japan.9
The molecular genetic basis of psoriasis is complex with evidence that multiple genes are involved. Seven major psoriasis susceptibility loci have been reported. Many investigators have established that a major susceptibility locus for psoriasis is at 6p21, referred to as PSORS1 and is overrepresented in all populations tested.10–15 As noted, an association between psoriasis and other loci has also been reported on chromosomes 1p (PSORS7),14 1q (PSORS4),16 3q (PSORS5),17 4q (PSORS3),18 17q (PSORS2),19 and 19p (PSORS6).20 The strength of associations between such genes and susceptibility to psoriasis, apart from PSORS1, is variable as replication of these findings has been incomplete. The difficulty of confirming psoriasis susceptibility loci may relate, in part, to heterogeneity among different populations. Whereas the existence of a genetic component in psoriasis is certain, the exact locations of the genes involved remains to be definitely determined.
CLINICAL FEATURES
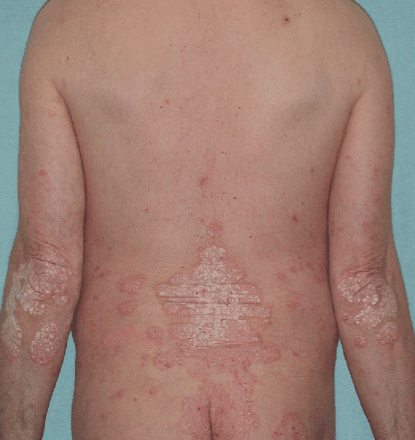
Psoriasis is a papulosquamous disease with variable morphology, distribution, severity, and course. Papulosquamous diseases are characterised by scaling papules (raised lesions <1 cm in diameter) and plaques (raised lesions >1 cm in diameter). Other papulosquamous diseases that may be considered in the differential diagnosis include tinea infections, pityriasis rosea, and lichen planus. The lesions of psoriasis are distinct from these other entities and are classically very well circumscribed, circular, red papules or plaques with a grey or silvery-white, dry scale. In addition, the lesions are typically distributed symmetrically on the scalp, elbows, knees, lumbosacral area, and in the body folds (fig 1). Psoriasis may also develop at the site of trauma or injury, known as Koebner’s phenomenon. If psoriasis is progressive or uncontrolled, it can result in a generalised exfoliative erythroderma. Nail involvement may be present, particularly if psoriatic arthritis (PsA) is present.
Occasionally psoriasis may involve the oral mucosa or the tongue. When the tongue is involved, the dorsal surface may have sharply circumscribed gyrate red patches with a white-yellow border. The patches may evolve and spread, changing on a daily basis, can assume distinct annular patterns and may resemble a map, hence the term geographic tongue.
Psoriasis can be highly variable in morphology, distribution, and severity. Despite the classic presentation described above, the morphology can range from small tear shaped papules (guttate psoriasis) to pustules (pustular psoriasis) and generalised erythema and scale (erythrodermic psoriasis). In addition, these different forms of psoriasis may be localised or widespread and disabling. Further, psoriasis may have a variable course presenting as chronic, stable plaques or may present acutely, with a rapid progression and widespread involvement. Psoriasis may be symptomatic with patients complaining of intense pruritus or burning. The various types and presentations of psoriasis are outlined below.
CLINICAL TYPES OF PSORIASIS
Plaque psoriasis
The commonest form of psoriasis is plaque psoriasis in which patients may have sharply circumscribed, round-oval, or nummular (coin-sized) plaques (fig 2). The lesions may initially begin as erythematous macules (flat and <1 cm) or papules, extend peripherally, and coalesce to form plaques of one to several centimetres in diameter. A white blanching ring, known as Woronoff’s ring, may be observed in the skin surrounding a psoriatic plaque. With gradual peripheral extension, plaques may develop different configurations including:
-
psoriasis gyrata—in which curved linear patterns predominate
-
annular psoriasis—in which ring-like lesions develop secondary to central clearing
-
psoriasis follicularis—in which minute scaly papules are present at the openings of pilosebaceous follicles.
The terms rupioid and ostraceous relate to distinct morphological subtypes of plaque psoriasis. Rupioid plaques are small (2–5 cm in diameter) and highly hyperkeratotic, resembling limpet shells. Ostraceous psoriasis refers to hyperkeratotic plaques with relatively concave centres, similar in shape to oyster shells.
Scale is typically present in psoriasis, is characteristically silvery white, and can vary in thickness. Removal of scale may reveal tiny bleeding points (Auspitz sign). The amount of scaling varies among patients and even at different sites on a given patient. In acute inflammatory or exanthematic psoriasis, scaling can be minimal and erythema may be the predominant clinical sign.
Guttate psoriasis
Guttate psoriasis, from the Greek word gutta meaning a droplet, describes the acute onset of a myriad of small, 2–10 mm diameter lesions of psoriasis. These are usually distributed in a centripetal fashion although guttate lesions can also involve the head and limbs. Classically, guttate psoriasis occurs shortly after an acute group B haemolytic streptococcal infection of the pharynx or tonsils and can be the presenting episode of psoriasis in children or, occasionally, adults. The number of lesions may range from five or 10 to over 100. Guttate psoriasis accounts for 2% of the total cases of psoriasis. In children, an acute episode of guttate psoriasis is usually self limiting; in adults, guttate flares may complicate chronic plaque disease. Although few studies have assessed the long term prognosis of children with acute guttate psoriasis, one small study revealed that 33% of patients with acute guttate psoriasis eventually developed chronic plaque disease.21
Flexural (inverse) psoriasis
Psoriasis affecting the flexures, particularly inframammary, perineal, and axillary, is distinct morphologically from traditional plaques elsewhere on the trunk and limbs. Flexural lesions are devoid of scale and appear as red, shiny, well demarcated plaques occasionally confused with candidal, intertrigo, and dermatophyte infections.
Erythroderma
Total or subtotal involvement of the skin by active psoriasis is known as erythroderma and may take one of two forms. Firstly, chronic plaque psoriasis may gradually progress as plaques become confluent and extensive. Secondly, erythroderma may be a manifestation of unstable psoriasis precipitated by infection, tar, drugs, or withdrawal of corticosteroids. Erythroderma may impair the thermoregulatory capacity of the skin, leading to hypothermia, high output cardiac failure, and metabolic changes including hypoalbuminaemia, and anaemia due to loss of iron, vitamin B12, and folate.
Generalised pustular psoriasis
Generalised pustular psoriasis (von Zumbusch) is rare and represents active, unstable disease. Precipitants include withdrawal of systemic or potent topical corticosteroids and infections. The patient is pyrexial, with red, painful, inflamed skin studded with monomorphic, sterile pustules, which may coalesce to form sheets. Patients with generalised pustular psoriasis frequently need to be admitted to the hospital for management.
Palmoplantar pustulosis
Palmoplantar pustulosis presents as sterile, yellow pustules on a background of erythema and scaling affecting the palms and/or soles (fig 3). The pustules are tender and fade to form dark brown coloration with adherent scale/crust. Palmoplantar pustulosis is frequently associated with psoriatic nail involvement. Approximately 25% of cases are associated with classic psoriasis vulgaris, but it is now believed that palmoplantar pustulosis may not be a form of psoriasis.22 This conclusion is derived from genetic studies showing no association with HLA-Cw6 or other markers on chromosome 6p—which are linked to chronic plaque and guttate psoriasis. The demographics of palmoplantar pustulosis are markedly different from those of chronic plaque psoriasis in that it more commonly affects women (9:1), presents most commonly between the ages of 40 and 60 years, and has a very striking association with smoking, either current or past, in up to 95% of subjects.23
Psoriatic nail disease
Fingernails are more commonly affected than toenails. The commonest finding is small pits in the nail plate, resulting from defective nail formation in the proximal portion of the nail matrix (fig 4). The nail may also detach from the bed at its distal or lateral attachments, known as onycholysis (see fig 4). Orange-yellow areas may be present beneath the nail plate and are termed “oil spots”. In addition, the nail plate may become, thickened, dystrophic, and discolored (fig 5). Yellow, keratinous material may collect under the nail plate and is known as subungual hyperkeratosis.
Nail plates in a patient with psoriasis. They are thickened, dystrophic, and show orange-yellow areas (oil spots).
QUALITY OF LIFE AND PSYCHOLOGICAL ASPECTS OF PSORIASIS
Although psoriasis generally does not affect survival, it certainly has a number of major negative effects on patients, demonstrable by a significant detriment to quality of life.24 Despite this, most clinical trials of new treatments for psoriasis focus on “objective” physical measures for the primary endpoint of efficacy. This is incongruous as it is the improvement in quality of life that patients and physicians rely upon when selecting treatment. Impairment of quality of life has been highlighted particularly by the work of Finlay.25,26 Patients with psoriasis have a reduction in their quality of life similar to or worse than patients with other chronic diseases, such as ischaemic heart disease and diabetes.25 That patients with psoriasis feel stigmatised by the condition is well established.30 This of itself contributes to everyday disability leading to depression and suicidal ideation in more than 5% of patients.28
Recent work has identified that pathological worry and anxiety occur in at least a third of patients with psoriasis and that psychological interpersonal difficulties impinge on all aspects of the patient’s daily life.29,31 The two main contributors to stress in patients with psoriasis are engaging in avoidance behaviour and the belief that they are being evaluated on the basis of their skin disease. This constraining, avoidance behaviour may lead to low grade persistent stress. Intriguingly, there is no significant relation between either the physical severity or anatomic location of psoriasis and psychological disability.32,33 This observation implies that “severity” of psoriasis is a composite of physical and psychological factors, a disparity further highlighted by the Psoriasis Disability Index.34 Stress in the form of pathological worry has a deleterious effect on response to therapy. For instance, in patients undergoing PUVA therapy, those who are delineated as being high or pathological worriers clear significantly more slowly, if at all, as compared with their counterparts who are low worriers.35 Psychological intervention may play a role in the management of psoriasis, particularly in the form of cognitive behavioural stress management.36 This form of intervention, when used as an adjunct to regular pharmacological therapy, produces a significant additional benefit identified as improvement in clinical severity of disease. How psychological distress exacerbates or triggers psoriasis is poorly understood. Up to 60% of patients describe stress as being a key “exacerbator” or trigger of their disease.8,37,38 It is known that psychological stress has the potential to regulate the immune response, and there is emerging evidence that abnormal neuroendocrine responses to stress may contribute to the pathogenesis of chronic autoimmune diseases, as has been described for rheumatoid arthritis (RA).39 It is likely that, in some patients with psoriasis, there is an abnormal hypothalamical–adrenal axis response to acute stress, undoubtedly an area deserving of further investigation.
Many instruments have been generated to measure aspects of disease on quality of life. Some reflect general health status, some reflect on skin disease in general, and yet others assess the impact of psoriasis and PsA (table 1). The current metrics for quality of life in psoriasis generally measure one or two categories, the physical aspects of disease (pain, itch, etc) or the mental aspects of disease (self perception, interaction with others, etc). To have a maximal quality of life, one needs to be able to participate in all aspects of life, including effective interaction with others and carrying out physical responsibilities, both at work and at home. Patient oriented quality of life measures are particularly beneficial in chronic diseases as they assess how the disease affects a person socially, psychologically, and physically.47
Furthermore, quality of life measures take into account the effect of the treatment on the patient. Quality of life data fulfils the role of measuring the intangible changes in a patient’s life that determine “treatment success”. For a clinically meaningful change to exist for psoriasis and other chronic, non-life threatening diseases, a treatment must provide an improvement in the patient’s quality of life. In an attempt to provide an holistic assessment of overall disease severity, a specific tool has been developed—the Salford Psoriasis Index (SPI)32:
-
S—Signs: a 0–10 measure of physical severity derived from the PASI
-
P—Psychosocial disability: measured as 0–10 on a visual analogue scale
-
I—Interventions: a cumulative historical record of systemic therapies, episodes of erythroderma, etc.
The SPI is represented as three figures such as 9,7,6 and is a guide to the difficulty of treating any one patient at a certain time.
Physicians evaluating chronic disease states, such as RA and inflammatory bowel disease (IBD), have used quality of life data to assess treatment efficacy. The Inflammatory Bowel Disease Questionnaire, a commonly used quality of life measure for IBD, has been validated in Crohn’s disease48 and has been shown to correlate highly with the commonly used objective measure, the Crohn’s Disease Activity Index (CDAI).49 The CDAI also incorporates a quality of life assessment, “the patient’s sense of wellbeing”, as one of the eight measurable items.50 In the American College of Rheumatology (ACR) improvement criteria for RA, a quality of life measure is often employed as the measure of disability.51 Moreover, ACR response rates have been found to be higher when quality of life criteria are used instead of objective measures, such as grip strength, to assess physical function/disability.52
For psoriasis, many quality of life instruments have been developed and tested in clinical trials to assess treatment response where the primary endpoint is the number of patients gaining a 75% reduction in the Psoriasis Area and Severity Index (PASI) relative to placebo. Table 1 lists these and a few elements of each. In a review of trials where both physical measures and quality of life were collected, two things stood out. First, the correlation with the physical measure, such as the PASI, and quality of life is generally very poor, the correlation coefficient being less than 0.2. Second, the improvement in quality of life over time generally parallels the physical measure.53 This supports the notion that quality of life and the PASI measure two different aspects of disease. Given that it is the promise of change in quality of life by a given treatment that patients and physician rely on in choosing treatment, it is not surprising that considerable thought and energy have gone into generating instruments that easily and reproducibly measure quality of life.
A number of instruments have been designed to generate disease specific quality of life assessments, of which several are represented in table 1. These offer advantages in that they house quality of life issues unique to that disease and hence would be more robust in following disease specific quality of life issues. Recently, McKenna and colleagues focused on generating a disease specific quality of life instrument by developing questions after an extensive interview process. Following this, a Rasch analysis was used to select questions that fit with quality of life issues for the test on test–retest. This approach led to 25 and 20 question profiles that appear to be specific to quality of life issues for patients with psoriatic arthritis45 and psoriasis,46 respectively. Whether these instruments will be more robust for quality of life in patients with psoriasis than those designed for general health or specific for skin disease or psoriasis remains to be determined.
Whereas the general health instruments, such as the SF-36 (see table 1), can be used to compare the burden of disease of different diseases such as diabetes and psoriasis, these instruments are not good at incorporating outcomes into cost effectiveness analysis. An instrument called “utilities” has been under development for this, and recently it has been applied to skin diseases. Utilities are measured in a manner that permits interpretation across diseases and populations. This is accomplished by asking patients to indicate their willingness to trade disease free status for the remainder of their lives in exchange for a reduction in their lifespan and to indicate the amount of reduction they would be willing to accept. As an example, patients in follow up for psoriasis indicated a willingness to trade 2.8 years of their remaining 35 years of expected lifespan for no disease. By extrapolation, patients with severe psoriasis would appear to be willing to trade 4.2 years for no disease, equivalent to that of a patient with metastatic cancer of the prostate.47
CONCLUSION
At this time, there are many instruments to measure quality of life for psoriasis and PsA. It does not appear that one will cover all the issues that quality of life encompasses. Additional testing is needed to better define which elements of quality of life are sensitive and predictive of clinically meaningful changes.
Instruments used in assessing quality of life in psoriasis and psoriatic arthritis
Symmetrical distribution of psoriatic lesions on the back and elbows.
Nummular (coin-sized) lesions of psoriasis.
Palmoplantar pustulosis.
Nail changes in psoriasis. Reproduced with permission.