Abstract
Significant advances have taken place in recent years in our understanding of the aetiopathogenesis, anagement, and clinical outcome of juvenile idiopathic arthritis (JIA). Fundamental to this advancement has been international collaborative efforts of the clinical scientific community and all those involved in the multidisciplinary care of children and young people with JIA.
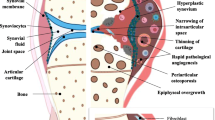
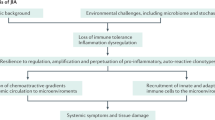
A key factor has been facing the challenge of developing a robust classification system for JIA, a clinically very heterogeneous group of conditions. JIA illustrates the necessity of disease classification to enable scientific progress but also the iterative and evolving process this entails. What is emerging is the imperative to improve our understanding of the biologic and genetic basis of JIA to underpin classification systems.
Growing emphasis is centered on improved holistic care and outcome of children and young people with JIA. The expectation of patients, their families, and clinicians is the goal of inactive disease, remission off treatment, and the health and psychosocial well-being of young people emerging into adulthood. Validated tools that reflect these challenges are being developed, including those measuring disease improvement, flare, remission and minimal disease activity, health-related quality of life, and composite scores of activity and damage.
Clinical research networks have driven success in developing an evidence-base for the treatment of JIA. Randomized comparative trials have demonstrated the benefit of early use of intra-articular corticosteroid injections, and the importance of methotrexate as the first-line, disease-modifying antirheumatic drug in JIA. The introduction of biologic therapies has opened a major new epoch in the medical management of JIA, with recent trials published on etanercept, infliximab, adalimumab, abatacept, tocilizumab, and anakinra.
This review focuses on recent advances in JIA, especially developments in its classification, validation of appropriate measures of holistic outcome, and the specific contribution of established and newer pharmacologic agents available for treating children and young people.



Similar content being viewed by others
References
Brough R, Cleary G. When does a knee “need” a “joint” assessment? Arch Dis Child Educ Pract Ed 2007; 92(2): ep44–9
Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004; 31(2): 390–2
Symmons DP, Jones M, Osborne J, et al. Pediatric rheumatology in the United Kingdom: data from the British Pediatric Rheumatology Group National Diagnostic Register. J Rheumatol 1996; 23(11): 1975–80
Beresford MW, Baildam EM. New advances in the management of juvenile idiopathic arthritis: I. Non-biological therapy. Arch Dis Child Educ Pract Ed 2009; 94(5): 144–50
Petty RE, Southwood TR, Baum J, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol 1998; 25(10): 1991–4
Foeldvari I, Bidde M. Validation of the proposed ILAR classification criteria for juvenile idiopathic arthritis. International League of Associations for Rheumatology. J Rheumatol 2000; 27(4): 1069–72
Fantini F. Classification of chronic arthritides of childhood (juvenile idiopathic arthritis): criticisms and suggestions to improve the efficacy of the Santiago-Durban criteria. J Rheumatol 2001; 28(2): 456–9
Hofer MF, Mouy R, Prieur AM. Juvenile idiopathic arthritides evaluated prospectively in a single center according to the Durban criteria. J Rheumatol 2001; 28(5): 1083–90
US Department of Health & Human Services. US Food and Drug Administration [online]. Available from URL: http://www.fda.gov [Accessed 2011 Mar 2]
European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical investigation of medicinal products for the treatment of juvenile idiopathic arthritis. CPMP/EWP/422/04 [online]. Available from URL: http://www.ema.europa.eu/pdfs/human/ewp/042204en.pdf [Accessed 2011 Jan 27]
Dannecker GE, Quartier P. Juvenile idiopathic arthritis: classification, clinical presentation and current treatments. Horm Res 2009; 72Suppl. 1: 4–12
Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet 2007; 369(9563): 767–78
Prahalad S, Glass DN. A comprehensive review of the genetics of juvenile idiopathic arthritis [abstract]. Pediatr Rheumatol Online J 2008; 6: 11
Berkun Y, Padeh S. Environmental factors and the geoepidemiology of juvenile idiopathic arthritis. Autoimmun Rev 2010; 9(5): A319–24
Ellis JA, Munro JE, Ponsonby AL. Possible environmental determinants of juvenile idiopathic arthritis. Rheumatology (Oxford) 2010; 49(3): 411–25
Petty RE. Growing pains: the ILAR classification of juvenile idiopathic arthritis. J Rheumatol 2001; 28(5): 927–8
Merino R, de Inocencio J, Garcia-Consuegra J. Evaluation of revised International League of Associations for Rheumatology classification criteria for juvenile idiopathic arthritis in Spanish children (Edmonton 2001). J Rheumatol 2005; 32(3): 559–61
Stabile A, Avallone L, Compagnone A, et al. Focus on juvenile idiopathic arthritis according to the 2001 Edmonton revised classification from the International League of Associations for Rheumatology: an Italian experience. Eur Rev Med Pharmacol Sci 2006; 10(5): 229–34
Minden K, Niewerth M. Juvenile idiopathic arthritis: clinical subgroups and classification. Z Rheumatol 2008; 67(2): 100, 102–6, 108–10
Behrens EM, Beukelman T, Cron RQ. Juvenile idiopathic arthritis classification criteria: loopholes and diagnosis software. J Rheumatol 2007; 34(1): 234; author reply 234-5
Berntson L, Damgard M, Andersson-Gare B, et al. HLA-B27 predicts a more extended disease with increasing age at onset in boys with juvenile idiopathic arthritis. J Rheumatol 2008; 35(10): 2055–61
Griffin TA, Barnes MG, Ilowite NT, et al. Gene expression signatures in polyarticular juvenile idiopathic arthritis demonstrate disease heterogeneity and offer a molecular classification of disease subsets. Arthritis Rheum 2009; 60(7): 2113–23
Muller L, Kellenberger CJ, Cannizzaro E, et al. Early diagnosis of temporomandibular joint involvement in juvenile idiopathic arthritis: a pilot study comparing clinical examination and ultrasound to magnetic resonance imaging. Rheumatology (Oxford) 2009; 48(6): 680–5
Magni-Manzoni S, Epis O, Ravelli A, et al. Comparison of clinical versus ultrasound-determined synovitis in juvenile idiopathic arthritis. Arthritis Rheum 2009; 61(11): 1497–504
Haslam KE, McCann LJ, Wyatt S, et al. The detection of subclinical synovitis by ultrasound in oligoarticular juvenile idiopathic arthritis: a pilot study. Rheumatology (Oxford) 2010; 49(1): 123–7
Pascoli L, Wright S, McAllister C, et al. Prospective evaluation of clinical and ultrasound findings in ankle disease in juvenile idiopathic arthritis: importance of ankle ultrasound. J Rheumatol 2010; 37(11): 2409–14
Damasio MB, Malattia C, Martini A, et al. Synovial and inflammatory diseases in childhood: role of new imaging modalities in the assessment of patients with juvenile idiopathic arthritis. Pediatr Radiol 2010; 40(6): 985–98
Miller E, Uleryk E, Doria AS. Evidence-based outcomes of studies addressing diagnostic accuracy of MRI of juvenile idiopathic arthritis. AJR Am J Roentgenol 2009; 192(5): 1209–18
McKay GM, Cox LA, Long BW. Imaging juvenile idiopathic arthritis: assessing the modalities. Radiol Technol 2010; 81(4): 318–27
Baildam E, Davidson J. BSPAR position statement on professionals working in paediatric rheumatology. Rheumatology (Oxford) 2008; 47(6): 743–4
Davies K, Cleary G, Foster H, et al. BSPAR standards of care for children and young people with juvenile idiopathic arthritis. Rheumatology (Oxford) 2010; 49(7): 1406–8
Wallace CA. Developing standards of care for patients with juvenile idiopathic arthritis. Rheumatology (Oxford) 2010; 49(7): 1213–4
Brunner HI, Ravelli A. Developing outcome measures for paediatric rheumatic diseases. Best Pract Res Clin Rheumatol 2009; 23(5): 609–24
Ringold S, Wallace CA. Measuring clinical response and remission in juvenile idiopathic arthritis. Curr Opin Rheumatol 2007; 19(5): 471–6
Ruperto N, Martini A. International research networks in pediatric rheumatology: the PRINTO perspective. Curr Opin Rheumatol 2004; 16(5): 566–70
Giannini EH, Ruperto N, Ravelli A, et al. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 1997; 40(7): 1202–9
Albornoz MA. ACR formally adopts improvement criteria for juvenile arthritis (ACR pediatric 30). ACR News 2002; 21: 3
Nugent J, Ruperto N, Grainger J, et al. The British version of the childhood health questionnaire (CHAQ) and the child health questionnaire (CHQ). Clin Exp Rheumatol 2001; 19Suppl. 23: S163–7
Ruperto N, Ravelli A, Falcini F, et al. Performance of the preliminary definition of improvement in juvenile chronic arthritis patients treated with methotrexate. Italian Pediatric Rheumatology Study Group. Ann Rheum Dis 1998; 57(1): 38–41
Brunner HI, Lovell DJ, Finck BK, et al. Preliminary definition of disease flare in juvenile rheumatoid arthritis. J Rheumatol 2002; 29(5): 1058–64
Wallace CA, Ruperto N, Giannini E. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol 2004; 31(11): 2290–4
Wallace CA, Ravelli A, Huang B, et al. Preliminary validation of clinical remission criteria using the OMERACT filter for select categories of juvenile idiopathic arthritis. J Rheumatol 2006; 33(4): 789–95
Wallace CA, Huang B, Bandeira M, et al. Patterns of clinical remission in select categories of juvenile idiopathic arthritis. Arthritis Rheum 2005; 52(11): 3554–62
Ruperto N, Lovell DJ, Quartier P, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet 2008; 372(9636): 383–91
Magni-Manzoni S, Ruperto N, Pistorio A, et al. Development and validation of a preliminary definition of minimal disease activity in patients with juvenile idiopathic arthritis. Arthritis Rheum 2008; 59(8): 1120–7
Oliveira S, Ravelli A, Pistorio A, et al. Proxy-reported health-related quality of life of patients with juvenile idiopathic arthritis. The Pediatric Rheumatology International Trials Organization Multinational Quality of Life Cohort Study. Arthritis Rheum 2007; 57(1): 35–43
Gutierrez-Suarez R, Pistorio A, Cespedes CA, et al. Health-related quality of life of patients with juvenile idiopathic arthritis coming from 3 different geographic areas. The PRINTO multinational quality of life cohort study. Rheumatology (Oxford) 2007; 46(2): 314–20
Varni JW, Seid M, Smith KT, et al. The PedsQL in pediatric rheumatology: reliability, validity, and responsiveness of the Pediatric Quality of Life Inventory Generic Core Scales and Rheumatology Module. Arthritis Rheum 2002; 46(3): 714–25
Varni JW, Burwinkle TM, Seid M, et al. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003; 3(6): 329–41
Consolaro A, Ruperto N, Bazso A, et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum 2009; 61(5): 658–66
Viola S, Felici E, Magni-Manzoni S, et al. Development and validation of a clinical index for assessment of long-term damage in juvenile idiopathic arthritis. Arthritis Rheum 2005; 52(7): 2092–102
Ravelli A. The time has come to include assessment of radiographic progression in juvenile idiopathic arthritis clinical trials. J Rheumatol 2008; 35(4): 553–7
Thornton J, Beresford MW, Clayton P. Improving the evidence base for treatment of juvenile idiopathic arthritis: the challenge and opportunity facing the MCRN/ARC Paediatric Rheumatology Clinical Studies Group. Rheumatology (Oxford) 2008; 47(5): 563–6
Eberhard BA. Comparison of the intraarticular effectiveness of triamcinolone hexacetonide and triamcinolone acetonide in treatment of juvenile rheumatoid arthritis. J Rheumatol 2004; 31(12): 2507–12
Zulian F. Triamcinolone acetonide and hexacetonide intra-articular treatment of symmetrical joints in juvenile idiopathic arthritis: a double-blind trial. Rheumatology (Oxford) 2004; 43(10): 1288–91
Zulian F. Comparison of intra-articular triamcinolone hexacetonide and triamcinolone acetonide in oligoarticular juvenile idiopathic arthritis. Rheumatology (Oxford) 2003; 42(10): 1254–9
Lepore L. Treatment of juvenile idiopathic arthritis with intra-articular triamcinolone hexacetonide: evaluation of clinical effectiveness correlated with circulating ANA and T gamma/delta + and B CD5+ lymphocyte populations of synovial fluid. Clin Exp Rheumatol 2002; 20(5): 719–22
Marti P. Factors influencing the efficacy of intra-articular steroid injections in patients with juvenile idiopathic arthritis. Eur J Pediatr 2008; 167(4): 425–30
Hashkes PJ, Laxer RM. Medical treatment of juvenile idiopathic arthritis. JAMA 2005; 294(13): 1671–84
Cleary AG, Murphy HD, Davidson JE. Intra-articular corticosteriod injections in juvenile idiopathic arthritis. Arch Dis Child 2003; 88: 192–6
Henter JI, Horne A, Arico M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007; 48(2): 124–31
Giannini EH. Methotrexate in resistant juvenile rheumatoid arthritis: results of the USA-USSR double-blind, placebo-controlled trial. The Pediatric Rheumatology Collaborative Study Group and The Cooperative Children’s Study Group. N Engl J Med 1992; 326(16): 1043–9
Woo P. Randomized, placebo-controlled, crossover trial of low-dose oral methotrexate in children with extended oligoarticular or systemic arthritis. Arthritis Rheum 2000; 43(8): 1849–57
Ramanan AV. Use of methotrexate in juvenile idiopathic arthritis. Arch Dis Child 2003; 88(3): 197–200
Ruperto N. A randomized trial of parenteral methotrexate comparing an intermediate dose with a higher dose in children with juvenile idiopathic arthritis who failed to respond to standard doses of methotrexate. Arthritis Rheum 2004; 50(7): 2191–201
Cespedes-Cruz A, Gutierrez-Suarez R, Pistorio A, et al. Methotrexate improves the health-related quality of life of children with juvenile idiopathic arthritis. Ann Rheum Dis 2008; 67(3): 309–14
Foell D, Frosch M, Schulze zur WA, et al. Methotrexate treatment in juvenile idiopathic arthritis: when is the right time to stop? Ann Rheum Dis 2004; 63(2): 206–8
Foell D, Wulffraat N, Wedderburn LR, et al. Methotrexate withdrawal at 6 vs 12 months in juvenile idiopathic arthritis in remission: a randomized clinical trial. JAMA 2010; 303(13): 1266–73
Hunter PJ, Nistala K, Jina N, et al. Biologic predictors of extension of oligoarticular juvenile idiopathic arthritis as determined from synovial fluid cellular composition and gene expression. Arthritis Rheum 2010; 62(3): 896–907
Van Rossum MA. Sulfasalazine in the treatment of juvenile chronic arthritis: a randomized, double-blind, placebo-controlled, multicenter study. Dutch Juvenile Chronic Arthritis Study Group. Arthritis Rheum 1998; 41(5): 808–16
Van Rossum MA. Long-term outcome of juvenile idiopathic arthritis following a placebo-controlled trial: sustained benefits of early sulfasalazine treatment. Ann Rheum Dis 2007; 66(11): 1518–24
Kremer JM. Rational use of new and existing disease-modifying agents in rheumatoid arthritis. Ann Intern Med 2001; 134(8): 695–706
Kelly A, Ramanan AV. Recognition and management ofmacrophage activation syndrome in juvenile arthritis. Curr Opin Rheumatol 2007; 19(5): 477–81
Foster H. Autologous haematopoeitic stem cell rescue (AHSCR) for severe rheumatic disease in children: guidance for BSPAR members. Executive summary. Rheumatology (Oxford) 2006; 45(12): 1570–1
Brinkman DM. Autologous stem cell transplantation in children with severe progressive systemic or polyarticular juvenile idiopathic arthritis: long-term follow-up of a prospective clinical trial. Arthritis Rheum 2007; 56(7): 2410–21
Abinun M, Flood TJ, Cant AJ, et al. Autologous T cell depleted haematopoietic stem cell transplantation in children with severe juvenile idiopathic arthritis in the UK (2000–2007). Mol Immunol 2009; 47(1): 46–51
Beresford MW, Baildam EM. New advances in the management of juvenile idiopathic arthritis: II. The era of biologicals. Arch Dis Child Educ Pract Ed 2009; 94(5): 151–6
Strand V, Kimberly R, Isaacs JD. Biologic therapies in rheumatology: lessons learned, future directions. Nat Rev Drug Discov 2007; 6(1): 75–92
Gartlehner G, Hansen RA, Jonas BL, et al. Biologics for the treatment of juvenile idiopathic arthritis: a systematic review and critical analysis of the evidence. Clin Rheumatol 2008; 27(1): 67–76
Ilowite NT. Update on biologics in juvenile idiopathic arthritis. Curr Opin Rheumatol 2008; 20(5): 613–8
Diak P, Siegel J, La GL, et al. Tumor necrosis factor alpha blockers and malignancy in children: forty-eight cases reported to the Food and Drug Administration. Arthritis Rheum 2010; 62(8): 2517–24
Lehman TJ. Should the Food and Drug Administration warning of malignancy in children receiving tumor necrosis factor alpha blockers change the way we treat children with juvenile idiopathic arthritis? Arthritis Rheum 2010; 62(8): 2183–4
McCroskery P, Wallace CA, Lovell DJ, et al. Summary of worldwide pediatric malignancies reported after exposure to etanercept. Pediatr Rheumatol Online J 2010; 8: 18
McCann LJ, Woo P. Biologic therapies in juvenile idiopathic arthritis: why and for whom? Acta Reumatol Port 2007; 32(1): 15–26
Finckh A. At the horizon of innovative therapy in rheumatology: new biologic agents. Curr Opin Rheumatol 2008; 20(3): 269–75
Lovell DJ, Giannini EH, Reiff A, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med 2000; 342(11): 763–9
Lovell DJ, Ruperto N, Goodman S, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med 2008; 359(8): 810–20
Ruperto N, Lovell DJ, Cuttica R, et al. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum 2007; 56(9): 3096–106
Yokota S, Imagawa T, Mori M, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet 2008; 371(9617): 998–1006
Lovell DJ, Reiff A, Ilowite NT, et al. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum 2008; 58(5): 1496–504
Prince FH. Long-term follow-up on effectiveness and safety of etanercept in JIA: the Dutch national register. Ann Rheum Dis 2009; 68(5): 635–41
Horneff G. Safety and efficacy of combination of etanercept and methotrexate compared to treatment with etanercept only in patients with juvenile idiopathic arthritis (JIA): preliminary data from the German JIA Registry. Ann Rheum Dis 2009; 68(4): 519–25
Vojvodich PF. Etanercept treatment improves longitudinal growth in prepubertal children with juvenile idiopathic arthritis. J Rheumatol 2007; 34(12): 2481–5
Gerloni V. Efficacy of repeated intravenous infusions of an anti-tumor necrosis factor alpha monoclonal antibody, infliximab, in persistently active, refractory juvenile idiopathic arthritis: results of an open-label prospective study. Arthritis Rheum 2005; 52(2): 548–53
Lahdenne P. Infliximab or etanercept in the treatment of children with refractory juvenile idiopathic arthritis: an open label study. Ann Rheum Dis 2003; 62(3): 245–7
Quartier P. Efficacy of etanercept for the treatment of juvenile idiopathic arthritis according to the onset type. Arthritis Rheum 2003; 48(4): 1093–101
Horneff G. The German etanercept registry for treatment of juvenile idiopathic arthritis. Ann Rheum Dis 2004; 63(12): 1638–44
European Medicines Agency [online]. Available from URL: www.ema.europa.eu/ema [Accessed 2011 Feb 9]
Woo P. Systemic juvenile idiopathic arthritis: diagnosis, management, and outcome. Nat Clin Pract Rheumatolol 2006; 2(1): 28–34
WooP. Open label phase II trial of single, ascending doses of MRA in Caucasian children with severe systemic juvenile idiopathic arthritis: proof of principle of the efficacy of IL-6 receptor blockade in this type of arthritis and demonstration of prolonged clinical improvement. Arthritis Res Ther 2005; 7(6): R1281–8
Hoffmann-La Roche. A study of tocilizumab in patients with active polyarticular-course juvenile idiopathic arthritis [ClinicalTrials.gov identifier NCT00988221]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov/ct2/show/NCT00988221 [Accessed 2011 Jan 28]
Lequerre T. Interleukin-1 receptor antagonist (anakinra) treatment in patients with systemic-onset juvenile idiopathic arthritis or adult onset Still disease: preliminary experience in France. Ann Rheum Dis 2008; 67(3): 302–8
Woo P. Anakinra treatment for systemic juvenile idiopathic arthritis and adult onset Still disease. Ann Rheum Dis 2008; 67(3): 281–2
Ohlsson V. Anakinra treatment for systemic onset juvenile idiopathic arthritis (SOJIA). Rheumatology (Oxford) 2008; 47(4): 555–6
Gattorno M. The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 2008; 58(5): 1505–15
Novartis Pharmaceuticals. Single-dose study to assess efficacy of canakinumab (ACZ885) in patients with active systemic juvenile idiopathic arthritis [SJIA] (β-SPECIFIC 1) [ClinicalTrials.gov identifier NCT00886769]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov/ct2/show/NCT00886769 [Accessed 2011 Jan 28]
Novartis Pharmaceuticals. Flare prevention study of canakinumab in patients with active systemic juvenile idiopathic arthritis [SJIA] (β-SPECIFIC 2) [ClinicalTrials.gov identifier NCT00889863]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov/ct2/show/NCT00889863 [Accessed 2011 Jan 28]
Novartis Pharmaceuticals. An open-label extension study of canakinumab in patients with systemic juvenile idiopathic arthritis and active systemic manifestations (β-SPECIFIC 3) [ClinicalTrials.gov identifier NCT00891046]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov/ct2/show/NCT00891046 [Accessed 2011 Jan 28]
Feldman BM. Treating children with arthritis: towards an evidence-based culture. J Rheumatol Suppl 2005; 72: 33–5
Medicines for Children Research Network [online]. Available from URL: http://mcrn.org.uk [Accessed 2011 Feb 9]
Acknowledgements
No sources of funding were used to prepare this review. The author has no conflicts of interest to declare that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beresford, M.W. Juvenile Idiopathic Arthritis. Pediatr-Drugs 13, 161–173 (2011). https://doi.org/10.2165/11588140-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11588140-000000000-00000