-
PDF
- Split View
-
Views
-
Cite
Cite
Zahi Touma, Dafna D. Gladman, Dominique Ibañez, Murray B. Urowitz, Ability of non-fasting and fasting triglycerides to predict coronary artery disease in lupus patients, Rheumatology, Volume 51, Issue 3, March 2012, Pages 528–534, https://doi.org/10.1093/rheumatology/ker339
Close - Share Icon Share
Abstract
Objectives. To test whether non-fasting and fasting triglyceride (TG) levels differ in individual patients and whether TG (non-fasting and fasting) levels predict coronary artery disease (CAD) in lupus patients.
Methods. Using predefined criteria for a patient's inclusion in this study, we identified the first available set of non-fasting and fasting TG measurements on each individual lupus patient seen in the clinic since 1996. We dichotomized TG values as normal/abnormal and determined whether non-fasting and fasting TG levels differ in each individual patient. We determined whether TG levels (non-fasting and fasting) predict CAD in all consecutive lupus patients seen in the clinic since 1973 using time-dependent time-to-event analysis and stepwise reduction analysis.
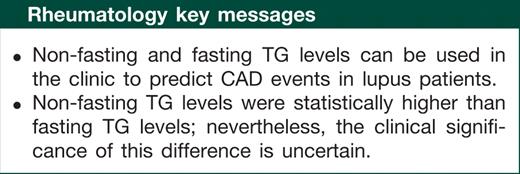
Results. Part 1: 514 patients were identified. The time between first non-fasting and fasting TG measurements available was 3.2 months. Examining dichotomized TG values as normal/abnormal, there was concordance between fasting and non-fasting TG in 92% of the visits. Non-fasting TG levels were 0.16 (0.75) higher than fasting TG levels (P < 0.001). Part 2: among 1289 patients, 638 had at least one elevated TG level and the length of follow-up from the first TG level recorded to CAD or last clinic visit was 8.82 years. One hundred and four patients developed CAD. TG (non-fasting and fasting) levels predicted CAD with a hazard ratio of 1.15 (95% CI 1.02, 1.29).
Conclusions. Although non-fasting TG levels were statistically higher than the fasting TG levels, the clinical significance of this difference is uncertain. TG (non-fasting and fasting) levels can predict CAD in lupus patients.
Introduction
Survival of patients with SLE has improved significantly over a 36-year period [1]. New morbidities have emerged and mortality in SLE follows a bimodal pattern [2]. Patients who die early in the course of their disease, die with active lupus and have a high incidence of infection. In those who die late in the course of the disease, death is associated with inactive lupus and a striking incidence of myocardial infarction due to atherosclerotic heart disease [2].
The prevalence of clinical coronary artery disease (CAD), angina and myocardial infarction, has ranged from 6 to 10% [3–7]. Compared with women in the general population, women with SLE have been found to be five to eight times more likely to develop CAD, with this risk being particularly marked in women <55 years of age [6, 7]. The recent results from a multinational inception cohort of SLE on 1249 patients followed for up to 8 years determined 31 events that were attributable to atherosclerosis; myocardial infarction (n = 13), angina (n = 15), congestive heart failure (n = 24), peripheral vascular disease (n = 8), transient ischaemic attack (n = 13), stroke (n = 23) and pacemaker insertion (n = 1) [8].
Several risk factors have been linked with the development of CAD in lupus patients including elevated total cholesterol, hypertension and older age at diagnosis of SLE [5, 6]. Studies have found that classic risk factors for atherosclerosis as defined in the Framingham model do not fully account for the increased risk of CAD in SLE [3–6, 9–11]. After controlling for traditional risk factors such as hypertension and hypercholesterolaemia, the relative risk of CAD events in patients with SLE is still over seven times that of controls [10]. Lupus-related factors that confer risk of CAD independently of traditional risk factors remain as previous cardiac involvement with SLE, and could be related to the disease itself, longer mean SLE duration and longer duration of CS use, and greater duration and use of CSs [3, 5, 6, 9, 12].
Hypertriglyceridaemia has been associated with cardiovascular disease, but its relation to atherosclerosis is unclear [13, 14]. Studies have shown that patients with moderate hypertriglyceridaemia and metabolic syndrome developed premature atherosclerosis [14, 15]. Moreover, elevated non-fasting triglyceride (TG) levels were associated with increased risk of myocardial infarction, ischaemic heart disease and death in men and women [14]. Researchers hypothesized that non-fasting TG levels indicate the presence of remnant lipoproteins, which may promote atherosclerosis [14]. Fasting TG levels increase the adjusted hazard ratios (HRs) for cardiovascular disease risk 1.7 times (comparing upper with lower tertile of TG levels), and non-fasting TG levels around 2.0 times. Moreover, non-fasting TG levels were linked with risk of ischaemic stroke [16]. Routinely, TGs are measured in the fasting state, after an 8–12 h fast, thus excluding remnant lipoproteins; nevertheless, studies have suggested that non-fasting TG levels may replace fasting levels in assessing cardiovascular risk once standard reference values have been established [14, 17]. The aims of this study were (i) to test whether non-fasting and fasting TG levels differ in individual lupus patients and (ii) to assess whether TG (non-fasting and fasting) levels predict CAD in lupus patients.
Methods
Patient selection and clinical and laboratory assessment
Patients from the University of Toronto Lupus Clinic were studied. All consecutive patients seen in the clinic between 1973 and 2009 were included. Patients with SLE (four or more ACR criteria or three ACR criteria plus a typical histological lesion of SLE on renal or skin biopsy) have been followed prospectively at the University of Toronto Lupus Clinic [18, 19]. Collection and storage of data at the lupus clinic were conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Board of the University Health Network. Signed informed consent was obtained from all patients at the lupus clinic. Patients attended the lupus clinic at 2- to 6-month intervals regardless of the state of activity of their lupus.
The standard protocol included: complete history, physical examination and laboratory evaluation. Disease activity was measured at each visit by the SLEDAI 2000 (SLEDAI-2K) 30 days, a valid measure of disease activity in SLE [20–22]. Organ damage was assessed using the SLICC/ACR damage index (SDI) [23]. This instrument records damage in 12 systems reflecting non-reversible accumulated damage since the onset of SLE, without attribution. All items have been recorded prospectively in the database. The SDI is completed once yearly. Written consent was obtained from all patients and the study was approved by the Institutional Review Board at the University of Toronto, Toronto Western Hospital.
Laboratory assessment
Fasting lipid profile (including fasting TG levels) were measured once yearly from 1991 and non-fasting lipid profile (including non-fasting TG levels) was determined at all other visits (at 2- to 6-month intervals) from 1973. Blood for fasting TG levels was obtained for laboratory analyses following a 14-h fast. Non-fasting TG levels are measured between 9 am and 12 pm, and between 1 and 5 pm during clinic hours. TG levels were measured in plasma using commercially available assays (Siemens Advia Chemistry, 2008 Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA).
Study design and statistical analysis
Our first aim was to determine the variability of fasting and non-fasting TG levels within each individual patient. We identified the first-available set of non-fasting and fasting TG measurements on each individual lupus patient seen in the clinic since 1996. The routine assessment of fasting TG levels was implemented in the clinic in 1991. We dichotomized TG values as normal/abnormal and determined whether non-fasting and fasting TG levels differ in individual patients. To control for the potential confounders, in particular change in disease activity and other patients’ demographics, we selected patients with two visits within 4 months, one who had fasting TG levels and one with non-fasting TG levels. Furthermore, their lipid-lowering therapy and steroid doses had to remain unchanged. We compared the fasting and non-fasting levels of TG using a paired t-test. We also dichotomized the values as normal/abnormal. Abnormal (borderline high values) plasma TG level was defined as >2.8 mmol/l for females and >3.3 mmol/l for males [24].
Our second aim was to determine whether TG (non-fasting and fasting) levels predict CAD in lupus patients. CAD was defined as angina or myocardial infarction. Comparison of potential risk factors for CAD was made for fasting and for non-fasting visits using repeated measures with generalized estimating equations. To assess whether the levels of TG (non-fasting and fasting) predict the risk of future CAD, we included the entire patient cohort since 1973 and all the values of TG (non-fasting and fasting) available in our database. We selected patients with no previous history of CAD. Time-dependent time-to-event analysis was conducted to determine the predictive ability of TGs for CAD—whether fasting and non-fasting. In the multivariate model, we adjusted for age, fasting TG (yes/no), disease duration, SDI, SLEDAI-2K, glucocorticoid, anti-malarial, immunosuppressive drugs and for traditional CAD risk factors in particular current smoking (one or more cigarettes/day), elevated total cholesterol (>5.2 mmol/l), hypertension (blood pressure ≥140 mmHg systolic or 90 mmHg diastolic on repeated measurements, or taking anti-hypertensive medication), diabetes mellitus (fasting plasma glucose >7.0 mmol/l, or current diabetic therapy) [24]. In the second model of TG level prediction for CAD, retained covariate were selected through the stepwise variable reduction strategy. We determined the HR for CAD for each increase by 0.5 mmol/l in TG (non-fasting and fasting) levels.
Results
Part 1
Between 1996 and 2007, a total of 514 patients were identified with a mean age at diagnosis of 29.4 (12.8) years, and the age and disease duration as of first TG available were 41.5 (14.1) and 12.0 (9.7) years, respectively. The majority of the 514 patients were females (90%). The patients’ ethnic distribution was Caucasian 65%, Black 13%, Asian 11% and others 10%.
Time between first-available TG level and follow-up visits was 3.2 (0.5) months. The disease activity determined by SLEDAI-2K as of first fasting TG level was 5.41 (5.34) and for first non-fasting TG level 5.12 (4.98) (P = 0.05). Steroids were taken by 68% of the patients. No statistically significant difference was present for other variables, in particular, SDI, current smoking, elevated total cholesterol, diabetes mellitus, anti-malarial and immunosuppressive drugs (Table 1).
Characteristics of the 514 patients evaluated to compare fasting and non-fasting TG levels taken 4 months apart
| Characteristic . | Fasting visit . | Non-fasting visit . | P-value . |
|---|---|---|---|
| Sex: female | 464 (90) | 464 (90) | NA |
| Race | |||
| Caucasian | 334 (65) | 334 (65) | NA |
| Black | 57 (13) | 57 (13) | |
| Asian | 59 (11) | 59 (11) | |
| Other | 54 (10) | 54 (10) | |
| Age at diagnosis, mean (s.d.), years | 29.4 (12.8) | 29.4 (12.8) | NA |
| Age, mean (s.d.), years | 41.5 (14.1) | 41.5 (14.1) | NA |
| Disease duration, mean (s.d.), years | 12.0 (9.7) | 12.0 (9.7) | NA |
| SLEDAI-2K, mean (s.d.) | 5.41 (5.34) | 5.12 (4.98) | 0.05 |
| SDI, mean (s.d.) | 1.54 (1.89) | 1.52 (1.86) | 0.33 |
| Current smoking | 73 (14.3) | 72 (14.1) | 0.71 |
| Steroids | 349 (67.9) | 349 (67.9) | 1.00 |
| Anti-malarials | 359 (69.8) | 350 (68.1) | 0.05 |
| Immunosuppressive | 249 (48.4) | 252 (49.0) | 0.51 |
| Elevated total cholesterol | 148 (28.8) | 136 (26.5) | 0.22 |
| Diabetes mellitus | 27 (5.3) | 31 (6.0) | 0.05 |
| Characteristic . | Fasting visit . | Non-fasting visit . | P-value . |
|---|---|---|---|
| Sex: female | 464 (90) | 464 (90) | NA |
| Race | |||
| Caucasian | 334 (65) | 334 (65) | NA |
| Black | 57 (13) | 57 (13) | |
| Asian | 59 (11) | 59 (11) | |
| Other | 54 (10) | 54 (10) | |
| Age at diagnosis, mean (s.d.), years | 29.4 (12.8) | 29.4 (12.8) | NA |
| Age, mean (s.d.), years | 41.5 (14.1) | 41.5 (14.1) | NA |
| Disease duration, mean (s.d.), years | 12.0 (9.7) | 12.0 (9.7) | NA |
| SLEDAI-2K, mean (s.d.) | 5.41 (5.34) | 5.12 (4.98) | 0.05 |
| SDI, mean (s.d.) | 1.54 (1.89) | 1.52 (1.86) | 0.33 |
| Current smoking | 73 (14.3) | 72 (14.1) | 0.71 |
| Steroids | 349 (67.9) | 349 (67.9) | 1.00 |
| Anti-malarials | 359 (69.8) | 350 (68.1) | 0.05 |
| Immunosuppressive | 249 (48.4) | 252 (49.0) | 0.51 |
| Elevated total cholesterol | 148 (28.8) | 136 (26.5) | 0.22 |
| Diabetes mellitus | 27 (5.3) | 31 (6.0) | 0.05 |
Values are expressed as n (%) unless otherwise noted. NA: not available.
Characteristics of the 514 patients evaluated to compare fasting and non-fasting TG levels taken 4 months apart
| Characteristic . | Fasting visit . | Non-fasting visit . | P-value . |
|---|---|---|---|
| Sex: female | 464 (90) | 464 (90) | NA |
| Race | |||
| Caucasian | 334 (65) | 334 (65) | NA |
| Black | 57 (13) | 57 (13) | |
| Asian | 59 (11) | 59 (11) | |
| Other | 54 (10) | 54 (10) | |
| Age at diagnosis, mean (s.d.), years | 29.4 (12.8) | 29.4 (12.8) | NA |
| Age, mean (s.d.), years | 41.5 (14.1) | 41.5 (14.1) | NA |
| Disease duration, mean (s.d.), years | 12.0 (9.7) | 12.0 (9.7) | NA |
| SLEDAI-2K, mean (s.d.) | 5.41 (5.34) | 5.12 (4.98) | 0.05 |
| SDI, mean (s.d.) | 1.54 (1.89) | 1.52 (1.86) | 0.33 |
| Current smoking | 73 (14.3) | 72 (14.1) | 0.71 |
| Steroids | 349 (67.9) | 349 (67.9) | 1.00 |
| Anti-malarials | 359 (69.8) | 350 (68.1) | 0.05 |
| Immunosuppressive | 249 (48.4) | 252 (49.0) | 0.51 |
| Elevated total cholesterol | 148 (28.8) | 136 (26.5) | 0.22 |
| Diabetes mellitus | 27 (5.3) | 31 (6.0) | 0.05 |
| Characteristic . | Fasting visit . | Non-fasting visit . | P-value . |
|---|---|---|---|
| Sex: female | 464 (90) | 464 (90) | NA |
| Race | |||
| Caucasian | 334 (65) | 334 (65) | NA |
| Black | 57 (13) | 57 (13) | |
| Asian | 59 (11) | 59 (11) | |
| Other | 54 (10) | 54 (10) | |
| Age at diagnosis, mean (s.d.), years | 29.4 (12.8) | 29.4 (12.8) | NA |
| Age, mean (s.d.), years | 41.5 (14.1) | 41.5 (14.1) | NA |
| Disease duration, mean (s.d.), years | 12.0 (9.7) | 12.0 (9.7) | NA |
| SLEDAI-2K, mean (s.d.) | 5.41 (5.34) | 5.12 (4.98) | 0.05 |
| SDI, mean (s.d.) | 1.54 (1.89) | 1.52 (1.86) | 0.33 |
| Current smoking | 73 (14.3) | 72 (14.1) | 0.71 |
| Steroids | 349 (67.9) | 349 (67.9) | 1.00 |
| Anti-malarials | 359 (69.8) | 350 (68.1) | 0.05 |
| Immunosuppressive | 249 (48.4) | 252 (49.0) | 0.51 |
| Elevated total cholesterol | 148 (28.8) | 136 (26.5) | 0.22 |
| Diabetes mellitus | 27 (5.3) | 31 (6.0) | 0.05 |
Values are expressed as n (%) unless otherwise noted. NA: not available.
Examining dichotomized TG values as normal/abnormal, there was concordance between fasting and non-fasting TG levels in 92% of the cases; only 40/514 (8%) were discordant. The mean fasting TG level [1.37 (0.78)] was statistically different from the mean non-fasting TG level [95% CI 1.54 (1.06)] (difference = 0.16 ± 0.75, P < 0.001), but this is of questionable clinical significance.
Part 2
For the time-to-event analysis, we identified 1289 patients from the date of the first TG (non-fasting and fasting) level available. One hundred and four (8.1%) patients developed CAD of all patients followed at the lupus clinic since 1973. CAD event occurred in 38 (5.2%) patients following fasting visits and 101 (7.9%) patients following non-fasting visits (P = 0.002). The length of follow-up from the first fasting visit available and first non-fasting visit available to CAD event or last clinic visit was 5.5 (3.7) and 8.8 (8.2) years, respectively (Table 2). Of 104 patients who had a CAD event, 20 (19%) patients had revascularization procedures. Patients with the fasting TG levels had longer disease duration and were older at the time of the first fasting TG level available and this is related to the fact that we started testing fasting TG in the lupus clinic in 1991, while results on non-fasting TG were available from 1973. This might also explain the statistically significant difference among fasting and non-fasting visits for SDI and the variables on treatment. Although there was a statistically significant difference among fasting and non-fasting visits for several variables as represented in Table 2, it is important to highlight that all TG levels, whether fasting or non-fasting, were included in the same model to predict CAD and that the model was able to distinguish whether TG level was fasting or not.
Demographics of 1289 patients evaluated to assess the capacity of fasting and non-fasting TG levels to predict CAD
| Characteristic . | Fasting visits . | Non-fasting visits . | P-value . |
|---|---|---|---|
| Number of patients | 724 | 1283 | NA |
| Number of visits | 2899 | 21 314 | |
| Number of CAD events, n (%) | 38 (5.2) | 101 (7.9) | 0.002 |
| Mean number of visits with TG level available before CAD/last visit, mean (s.d.) | 4.0 (2.8) | 16.6 (18.3) | |
| Time between visits, mean (s.d.), monthsa | 18.8 (13.0) | 8.0 (10.9) | |
| Length of follow-up from first TG level to CAD/last visit, mean (s.d.), years | 5.5 (3.7) | 8.8 (8.2) | |
| Sex: female,bn (%) | 652 (90.1) | 1137 (88.6) | 0.08 |
| Age at diagnosis, mean (s.d.), yearsb | 29.3 (13.1) | 30.7 (13.7) | <0.0001 |
| Age, mean (s.d.), yearsb | 39.2 (14.0) | 34.9 (13.5) | <0.0001 |
| Disease duration, mean (s.d.), yearsb | 10.0 (9.0) | 4.3 (5.8) | <0.0001 |
| SDI, mean (s.d.)a | 1.52 (1.79) | 1.12 (1.60) | <0.0001 |
| SLEDAI-2K, mean (s.d.)a | 4.76 (4.97) | 5.21 (5.06) | 0.001 |
| Current smoking,a % | 11.8 | 18.2 | <0.0001 |
| Steroids,a % | 61.5 | 66.0 | 0.003 |
| Anti-malarials,a % | 63.9 | 49.8 | <0.0001 |
| Immunosuppressive,a % | 44.6 | 31.2 | <0.0001 |
| Elevated total cholesterol,a % | 34.4 | 36.1 | 0.23 |
| Diabetes mellitus,a % | 5.9 | 4.6 | 0.07 |
| Characteristic . | Fasting visits . | Non-fasting visits . | P-value . |
|---|---|---|---|
| Number of patients | 724 | 1283 | NA |
| Number of visits | 2899 | 21 314 | |
| Number of CAD events, n (%) | 38 (5.2) | 101 (7.9) | 0.002 |
| Mean number of visits with TG level available before CAD/last visit, mean (s.d.) | 4.0 (2.8) | 16.6 (18.3) | |
| Time between visits, mean (s.d.), monthsa | 18.8 (13.0) | 8.0 (10.9) | |
| Length of follow-up from first TG level to CAD/last visit, mean (s.d.), years | 5.5 (3.7) | 8.8 (8.2) | |
| Sex: female,bn (%) | 652 (90.1) | 1137 (88.6) | 0.08 |
| Age at diagnosis, mean (s.d.), yearsb | 29.3 (13.1) | 30.7 (13.7) | <0.0001 |
| Age, mean (s.d.), yearsb | 39.2 (14.0) | 34.9 (13.5) | <0.0001 |
| Disease duration, mean (s.d.), yearsb | 10.0 (9.0) | 4.3 (5.8) | <0.0001 |
| SDI, mean (s.d.)a | 1.52 (1.79) | 1.12 (1.60) | <0.0001 |
| SLEDAI-2K, mean (s.d.)a | 4.76 (4.97) | 5.21 (5.06) | 0.001 |
| Current smoking,a % | 11.8 | 18.2 | <0.0001 |
| Steroids,a % | 61.5 | 66.0 | 0.003 |
| Anti-malarials,a % | 63.9 | 49.8 | <0.0001 |
| Immunosuppressive,a % | 44.6 | 31.2 | <0.0001 |
| Elevated total cholesterol,a % | 34.4 | 36.1 | 0.23 |
| Diabetes mellitus,a % | 5.9 | 4.6 | 0.07 |
aOverall of the available visits; bas of date of first visit available. NA: not available.
Demographics of 1289 patients evaluated to assess the capacity of fasting and non-fasting TG levels to predict CAD
| Characteristic . | Fasting visits . | Non-fasting visits . | P-value . |
|---|---|---|---|
| Number of patients | 724 | 1283 | NA |
| Number of visits | 2899 | 21 314 | |
| Number of CAD events, n (%) | 38 (5.2) | 101 (7.9) | 0.002 |
| Mean number of visits with TG level available before CAD/last visit, mean (s.d.) | 4.0 (2.8) | 16.6 (18.3) | |
| Time between visits, mean (s.d.), monthsa | 18.8 (13.0) | 8.0 (10.9) | |
| Length of follow-up from first TG level to CAD/last visit, mean (s.d.), years | 5.5 (3.7) | 8.8 (8.2) | |
| Sex: female,bn (%) | 652 (90.1) | 1137 (88.6) | 0.08 |
| Age at diagnosis, mean (s.d.), yearsb | 29.3 (13.1) | 30.7 (13.7) | <0.0001 |
| Age, mean (s.d.), yearsb | 39.2 (14.0) | 34.9 (13.5) | <0.0001 |
| Disease duration, mean (s.d.), yearsb | 10.0 (9.0) | 4.3 (5.8) | <0.0001 |
| SDI, mean (s.d.)a | 1.52 (1.79) | 1.12 (1.60) | <0.0001 |
| SLEDAI-2K, mean (s.d.)a | 4.76 (4.97) | 5.21 (5.06) | 0.001 |
| Current smoking,a % | 11.8 | 18.2 | <0.0001 |
| Steroids,a % | 61.5 | 66.0 | 0.003 |
| Anti-malarials,a % | 63.9 | 49.8 | <0.0001 |
| Immunosuppressive,a % | 44.6 | 31.2 | <0.0001 |
| Elevated total cholesterol,a % | 34.4 | 36.1 | 0.23 |
| Diabetes mellitus,a % | 5.9 | 4.6 | 0.07 |
| Characteristic . | Fasting visits . | Non-fasting visits . | P-value . |
|---|---|---|---|
| Number of patients | 724 | 1283 | NA |
| Number of visits | 2899 | 21 314 | |
| Number of CAD events, n (%) | 38 (5.2) | 101 (7.9) | 0.002 |
| Mean number of visits with TG level available before CAD/last visit, mean (s.d.) | 4.0 (2.8) | 16.6 (18.3) | |
| Time between visits, mean (s.d.), monthsa | 18.8 (13.0) | 8.0 (10.9) | |
| Length of follow-up from first TG level to CAD/last visit, mean (s.d.), years | 5.5 (3.7) | 8.8 (8.2) | |
| Sex: female,bn (%) | 652 (90.1) | 1137 (88.6) | 0.08 |
| Age at diagnosis, mean (s.d.), yearsb | 29.3 (13.1) | 30.7 (13.7) | <0.0001 |
| Age, mean (s.d.), yearsb | 39.2 (14.0) | 34.9 (13.5) | <0.0001 |
| Disease duration, mean (s.d.), yearsb | 10.0 (9.0) | 4.3 (5.8) | <0.0001 |
| SDI, mean (s.d.)a | 1.52 (1.79) | 1.12 (1.60) | <0.0001 |
| SLEDAI-2K, mean (s.d.)a | 4.76 (4.97) | 5.21 (5.06) | 0.001 |
| Current smoking,a % | 11.8 | 18.2 | <0.0001 |
| Steroids,a % | 61.5 | 66.0 | 0.003 |
| Anti-malarials,a % | 63.9 | 49.8 | <0.0001 |
| Immunosuppressive,a % | 44.6 | 31.2 | <0.0001 |
| Elevated total cholesterol,a % | 34.4 | 36.1 | 0.23 |
| Diabetes mellitus,a % | 5.9 | 4.6 | 0.07 |
aOverall of the available visits; bas of date of first visit available. NA: not available.
In the multivariate model analysis, TG (non-fasting and fasting) levels predicted CAD with an HR of 1.15 (95% CI 1.02, 1.29) as well as age, SLEDAI-2K, hypertension and elevated total cholesterol, with an HR of 1.06, 1.07, 1.70 and 1.64, respectively. The ability of TG levels to predict CAD was not altered by fasting TG (yes/no) (P = 0.06) levels. In the stepwise prediction model, the same variable as above showed a statistically significant ability to predict CAD and the use of immunosuppressives emerged as a new variable with an HR of 1.71 (95% CI 1.09, 2.66) (Table 3). The HRs for CAD were 1.23 (95% CI 1.03, 1.46) and 1.32 (95% CI 1.05, 1.65) for 1.5 and 2 mmol/l increase in TG levels, respectively (Table 4).
HR for CAD
| . | Model with all of the variables . | Stepwise model . | ||
|---|---|---|---|---|
| Variable . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| TG value | 1.15 (1.02, 1.29) | 0.02 | 1.15 (1.04, 1.28) | 0.009 |
| Fasting (Y/N) | 1.67 (0.98, 2.86) | 0.06 | ||
| Age | 1.06 (1.04, 1.08) | <0.0001 | 1.06 (1.04, 1.08) | <0.0001 |
| Disease duration | 1.01 (0.98, 1.04) | 0.44 | ||
| SDI | 1.07 (0.96, 1.04) | 0.24 | ||
| SLEDAI-2K | 1.07 (1.03, 1.11) | 0.0005 | 1.08 (1.04, 1.12) | <0.0001 |
| Current smoking (Y/N) | 1.58 (0.96, 2.63) | 0.08 | ||
| Hypertension (Y/N) | 1.70 (1.07, 2.70) | 0.02 | 1.77 (1.12, 2.80) | 0.02 |
| Elevated total cholesterol (Y/N) | 1.64 (1.05, 2.55) | 0.03 | 1.65 (1.07, 2.54) | 0.02 |
| Diabetes (Y/N) | 1.20 (0.60, 2.40) | 0.61 | ||
| Steroids (Y/N) | 1.54 (0.92, 2.60) | 0.10 | ||
| Anti-malarials (Y/N) | 1.01 (0.65, 1.57) | 0.97 | ||
| Immunosuppressive (Y/N) | 1.39 (0.86, 2.25) | 0.18 | 1.71 (1.09, 2.66) | 0.02 |
| . | Model with all of the variables . | Stepwise model . | ||
|---|---|---|---|---|
| Variable . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| TG value | 1.15 (1.02, 1.29) | 0.02 | 1.15 (1.04, 1.28) | 0.009 |
| Fasting (Y/N) | 1.67 (0.98, 2.86) | 0.06 | ||
| Age | 1.06 (1.04, 1.08) | <0.0001 | 1.06 (1.04, 1.08) | <0.0001 |
| Disease duration | 1.01 (0.98, 1.04) | 0.44 | ||
| SDI | 1.07 (0.96, 1.04) | 0.24 | ||
| SLEDAI-2K | 1.07 (1.03, 1.11) | 0.0005 | 1.08 (1.04, 1.12) | <0.0001 |
| Current smoking (Y/N) | 1.58 (0.96, 2.63) | 0.08 | ||
| Hypertension (Y/N) | 1.70 (1.07, 2.70) | 0.02 | 1.77 (1.12, 2.80) | 0.02 |
| Elevated total cholesterol (Y/N) | 1.64 (1.05, 2.55) | 0.03 | 1.65 (1.07, 2.54) | 0.02 |
| Diabetes (Y/N) | 1.20 (0.60, 2.40) | 0.61 | ||
| Steroids (Y/N) | 1.54 (0.92, 2.60) | 0.10 | ||
| Anti-malarials (Y/N) | 1.01 (0.65, 1.57) | 0.97 | ||
| Immunosuppressive (Y/N) | 1.39 (0.86, 2.25) | 0.18 | 1.71 (1.09, 2.66) | 0.02 |
HR for CAD
| . | Model with all of the variables . | Stepwise model . | ||
|---|---|---|---|---|
| Variable . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| TG value | 1.15 (1.02, 1.29) | 0.02 | 1.15 (1.04, 1.28) | 0.009 |
| Fasting (Y/N) | 1.67 (0.98, 2.86) | 0.06 | ||
| Age | 1.06 (1.04, 1.08) | <0.0001 | 1.06 (1.04, 1.08) | <0.0001 |
| Disease duration | 1.01 (0.98, 1.04) | 0.44 | ||
| SDI | 1.07 (0.96, 1.04) | 0.24 | ||
| SLEDAI-2K | 1.07 (1.03, 1.11) | 0.0005 | 1.08 (1.04, 1.12) | <0.0001 |
| Current smoking (Y/N) | 1.58 (0.96, 2.63) | 0.08 | ||
| Hypertension (Y/N) | 1.70 (1.07, 2.70) | 0.02 | 1.77 (1.12, 2.80) | 0.02 |
| Elevated total cholesterol (Y/N) | 1.64 (1.05, 2.55) | 0.03 | 1.65 (1.07, 2.54) | 0.02 |
| Diabetes (Y/N) | 1.20 (0.60, 2.40) | 0.61 | ||
| Steroids (Y/N) | 1.54 (0.92, 2.60) | 0.10 | ||
| Anti-malarials (Y/N) | 1.01 (0.65, 1.57) | 0.97 | ||
| Immunosuppressive (Y/N) | 1.39 (0.86, 2.25) | 0.18 | 1.71 (1.09, 2.66) | 0.02 |
| . | Model with all of the variables . | Stepwise model . | ||
|---|---|---|---|---|
| Variable . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| TG value | 1.15 (1.02, 1.29) | 0.02 | 1.15 (1.04, 1.28) | 0.009 |
| Fasting (Y/N) | 1.67 (0.98, 2.86) | 0.06 | ||
| Age | 1.06 (1.04, 1.08) | <0.0001 | 1.06 (1.04, 1.08) | <0.0001 |
| Disease duration | 1.01 (0.98, 1.04) | 0.44 | ||
| SDI | 1.07 (0.96, 1.04) | 0.24 | ||
| SLEDAI-2K | 1.07 (1.03, 1.11) | 0.0005 | 1.08 (1.04, 1.12) | <0.0001 |
| Current smoking (Y/N) | 1.58 (0.96, 2.63) | 0.08 | ||
| Hypertension (Y/N) | 1.70 (1.07, 2.70) | 0.02 | 1.77 (1.12, 2.80) | 0.02 |
| Elevated total cholesterol (Y/N) | 1.64 (1.05, 2.55) | 0.03 | 1.65 (1.07, 2.54) | 0.02 |
| Diabetes (Y/N) | 1.20 (0.60, 2.40) | 0.61 | ||
| Steroids (Y/N) | 1.54 (0.92, 2.60) | 0.10 | ||
| Anti-malarials (Y/N) | 1.01 (0.65, 1.57) | 0.97 | ||
| Immunosuppressive (Y/N) | 1.39 (0.86, 2.25) | 0.18 | 1.71 (1.09, 2.66) | 0.02 |
HR for CAD for each 0.5 mmol/l increase in TG (non-fasting and fasting) levels
| . | HR . | P-value . |
|---|---|---|
| Increase by 0.5 | 1.07 (1.01, 1.13) | 0.02 |
| Increase by 1.0 | 1.15 (1.02, 1.29) | 0.02 |
| Increase by 1.5 | 1.23 (1.03, 1.46) | 0.02 |
| Increase by 2 | 1.32 (1.05, 1.65) | 0.02 |
| . | HR . | P-value . |
|---|---|---|
| Increase by 0.5 | 1.07 (1.01, 1.13) | 0.02 |
| Increase by 1.0 | 1.15 (1.02, 1.29) | 0.02 |
| Increase by 1.5 | 1.23 (1.03, 1.46) | 0.02 |
| Increase by 2 | 1.32 (1.05, 1.65) | 0.02 |
HR for CAD for each 0.5 mmol/l increase in TG (non-fasting and fasting) levels
| . | HR . | P-value . |
|---|---|---|
| Increase by 0.5 | 1.07 (1.01, 1.13) | 0.02 |
| Increase by 1.0 | 1.15 (1.02, 1.29) | 0.02 |
| Increase by 1.5 | 1.23 (1.03, 1.46) | 0.02 |
| Increase by 2 | 1.32 (1.05, 1.65) | 0.02 |
| . | HR . | P-value . |
|---|---|---|
| Increase by 0.5 | 1.07 (1.01, 1.13) | 0.02 |
| Increase by 1.0 | 1.15 (1.02, 1.29) | 0.02 |
| Increase by 1.5 | 1.23 (1.03, 1.46) | 0.02 |
| Increase by 2 | 1.32 (1.05, 1.65) | 0.02 |
Discussion
In this study, we found a minimal difference between non-fasting and fasting TG levels in lupus patients. Indeed, non-fasting TG levels varied only with a mean increase of 0.16 as compared with fasting TG levels. Secondly, we showed that TG levels predicted CAD events in our lupus cohort and, more importantly, the ability of TG levels to predict CAD was not altered by fasting TG (yes/no) (P = 0.06).
Routinely, TGs are measured in the fasting state, after 8–12 h of fasting, thus excluding remnant lipoproteins. Nevertheless, studies have suggested that non-fasting TG levels may replace fasting levels in assessing cardiovascular risk once standard reference values have been established [14, 17, 25–27]. The Copenhagen General Population Study and the Copenhagen City Heart Study showed that after normal food intake, individuals in the general population had a mean change from fasting levels of 0.3 mmol/l for TGs at 1–4 h after the last meal [28]. In our study, patients were seen between 9 am and 12 pm, and between 1 and 5 pm. Thus, the non-fasting TGs were measured either after breakfast or lunch. In both cases, this ranged between 1 and 4 h after the last meal, similar to the Copenhagen study. Indeed, this time frame will be the most commonly encountered if one assessed non-fasting TG levels in the clinic. Furthermore, measurement of non-fasting TG levels is more feasible and much more convenient for the patients. At the same time, non-fasting TG levels could provide more information on remnant lipoproteins and could be more predictive of cardiovascular disease as compared with fasting TG levels [17]. Other researchers suggested that a TG tolerance test using a standardized meal that is analogous to glucose tolerance test needs to be developed [25].
Several factors, including diet composition, gender, apolipoprotein E genotype, age, menopausal status, glycaemic status and visceral fat deposition, have been shown to influence non-fasting TG levels. A recent study reported that waist circumference was a significant determinant of non-fasting TG levels. This indicates that subjects with a larger waist circumference may have higher levels of non-fasting triglycerides [26]. Unfortunately, in our study we could not study the implication of waist circumference and BMI on fasting and non-fasting TG levels because of lack of data. Langsted et al. [28] further detected only minimal changes in levels of lipids, lipoproteins and apolipoproteins in response to normal food intake. Furthermore, Langsted et al. [28] showed that non-fasting TG levels also predict cardiovascular events and they challenge the necessity of determining fasting TG levels for cardiovascular risk prediction [28].
Post-prandial hypertriglyceridaemia is recognized as an independent risk factor for cardiovascular disease [14, 25–28]. In our study, we showed that non-fasting and fasting TG levels predicted CAD in lupus patients. Furthermore, we confirmed a trend towards an increase in the HR for CAD with the increase in the level of TGs. Data from large prospective studies in Western populations point to moderate and highly significant associations between TG values and coronary heart disease risk [27]. A long-term prospective cohort study with a mean follow-up of 26 years and almost 14 000 patients shows that elevated non-fasting TG levels predicted cardiovascular events including myocardial infarction, ischaemic heart disease and death in men and women [14]. In addition, the authors demonstrated that remnant lipoproteins are a function of serum non-fasting TGs, which may explain the increased risk [14]. Results from a second long-term prospective cohort study, with a median follow-up of 11.4 years, showed that non-fasting TG levels were strongly associated with cardiovascular events as compared with fasting TG levels, which showed a minor independent relationship with cardiovascular risk [25]. In another study, non-fasting TG levels were found to be associated with the risk of ischaemic stroke [16].
Our study has few limitations. One of the major limitations is the absence of accurate information regarding the time when patients had eaten their last meal before blood sampling. However, as mentioned before, blood samples were collected ∼1–4 h after meals and this range of time has been used by previous studies on non-fasting TG levels [28]. The second limitation is that we did not draw blood from the same individuals in the fasting state and at shorter intervals after the last meals. There was a time delay of 1–3 months between the fasting and non-fasting levels of TG. Our results showed that non-fasting TG levels were 0.16 higher than fasting TG levels and this was statistically significant. Nevertheless, we interpreted the difference in the levels of fasting and non-fasting TGs as not clinically significant. This conclusion should be interpreted with caution given the shortcomings in the assessment of TG levels in our study as described in the first and second limitations. The third limitation is that we had no record of what patients had eaten before the blood test. This limitation needs to be addressed in future studies that would also evaluate the proposed standardized triglyceride tolerance test similar to glucose tolerance test. Our sample size was limited to 1283 patients in the non-fasting model and 724 in the fasting model; however, this was enough to predict CAD and demonstrate an increase in the HR with each 0.5 mmol/l increase in the levels of TGs.
In our study, we did not evaluate the influence of disease activity on TG levels. Our patients displayed mild to moderate disease activity [SLEDAI-2K = 4.8 (5.0) in fasting visits and 5.2 (5.1) in non-fasting visits]. Furthermore, previous studies have noted that dyslipoproteinaemia occurs in conjunction with active disease and is characterized by elevated levels of TG [4, 29, 30]. Additionally, compared with controls, regardless of disease activity overall lupus patients were found to have higher levels of TG [30, 31]. Studies have also shown that steroid-treated patients have higher levels of TGs. In Part 2 of our study, 65% of the patients were on steroids at enrolment. These numbers highlight the necessity and the importance of evaluating lupus patients for possible abnormal TG levels.
In this study, we wanted to assess the importance of TG levels (fasting and non-fasting) in predicting CAD in lupus patients. We showed that hypertriglyceridaemia is a metabolic disorder associated with CAD in lupus patients. We found that non-fasting TG levels are greater than fasting TG levels by 0.16 and this is of uncertain clinical significance. More importantly, measuring non-fasting TG levels can be used in the clinic to predict CAD events in lupus patients. Further studies are needed to evaluate the importance of treating high levels of TGs in lupus patients to prevent of CAD events.
Acknowledgements
Dr Zahi Touma is a recipient of the Lupus Ontario Geoff Carr Fellowship and the University of Toronto Arthritis Centre of Excellence Fellowship. The Lupus Clinic is supported by The Lupus Flare Foundation, Arthritis and Autoimmune Centre Foundation, Toronto General-Toronto Western Hospital Foundation and Smythe Foundation.
Disclosure statement: The authors have declared no conflicts of interest.




Comments