-
PDF
- Split View
-
Views
-
Cite
Cite
A. J. W. Zendman, W. J. van Venrooij, G. J. M. Pruijn, Use and significance of anti-CCP autoantibodies in rheumatoid arthritis, Rheumatology, Volume 45, Issue 1, January 2006, Pages 20–25, https://doi.org/10.1093/rheumatology/kei111
Close - Share Icon Share
One of the most common autoimmune diseases is rheumatoid arthritis (RA), affecting 0.5–1% of the population. This systemic disease is marked by chronic inflammation of synovial joints, which leads to destruction of cartilage and bone and eventually to disability of the patient [1]. Though not directly life-threatening, RA severely affects the quality of life of a patient and also has major economic consequences for society. Therefore, every attempt should be made to prevent the erosive processes to occur. Currently, the classification of RA relies mainly on the criteria described by the American College of Rheumatology (ACR) [2]. These criteria, originally formulated 50 yr ago and last adjusted in 1987, are based mainly on clinical parameters. Since these parameters are often only sufficiently fulfilled when the damaging effects of the inflammatory process are already in progress, this set of criteria is not very suitable for the early diagnosis of RA [3].
The key to early recognition of autoimmunity lies within the humoral immune system. Since blood samples are taken from clinic-visiting (pre-)patients, screening for serological RA-indicators can be performed quite easily. In the ACR criteria for RA one serological marker is included: rheumatoid factor (RF). The RF autoantibody system, directed against the Fc part of immunoglobulin (Ig) G molecules, has had a central role in the diagnosis and prognosis of RA during recent decades [4] because RF can be detected in the majority of RA patients. However, it is becoming more and more clear that the presence of RF is not restricted to patients with RA, but that it can also be detected in subsets of patients suffering from other diseases and even in a percentage of healthy (especially elderly) individuals [5]. The resulting lack of specificity for RA can lead to confusion and unwanted treatment. The shortcomings of the RF test have kept the search for more specific RA markers alive. Most autoantibody systems described during recent decades have failed to mature into mainstream tests for RA because of low sensitivity, lack of specificity or technical inconvenience, as reviewed previously [6–8]. The only antibody system that combines good sensitivity with superior specificity for RA is that targeting citrullinated epitopes. This review will focus mainly on the diagnostic potential of the second-generation anti-cyclic citrullinated peptide (CCP) test (CCP2) for RA. Next, we will address the prognostic ability of the anti-CCP test. The presence of these antibodies early in disease development opens a window of opportunity for early custom-tailored treatment of RA. Finally, we will review the effect of disease treatment on anti-CCP levels and briefly go into the putative functional role of these antibodies in the chronic aspects of RA.
From APF to CCP2
The autoantibody system most specific for RA known to date is that directed to citrullinated antigens. The citrulline moiety, which is the essential part of the antigenic determinant in these antigens, is post-translationally generated by peptidylarginine deiminases (PAD; EC 3.5.3.15) [9]. RA autoantibodies against citrullinated antigens have been detected and used for diagnostic purposes for many decades via the well-known antiperinuclear factor (APF) [10] and antikeratin antibody (AKA) tests [11]. Supported by the fact that APF and AKA share many features, as reviewed previously [12], and are reactive with native filaggrin, these autoantibodies are now designated as antifilaggrin antibodies (AFA) [13]. Using the laborious and inconvenient immunofluorescence assay, roughly 50% of RA sera can be scored AFA-positive. A key finding was the discovery that the reactivity of these AFA was completely dependent on the presence of citrulline residues (present in mature filaggrin but not in profilaggrin) [14, 15]. Since then, two approaches for detecting autoantibodies to citrullinated epitopes have been taken: a protein-based and a peptide-based approach.
Screening for citrulline-specific RA reactivity has been performed with several proteins, including both purified naturally occurring citrullinated proteins and in vitro-citrullinated proteins. For these purposes, mainly filaggrin, fibrinogen and myelin basic protein have been used. Although most of these proteins are arginine-rich, there is obviously a limit to the number of citrullinated epitopes associated with a certain protein. A complication of the use of natural antigens is that it is difficult to obtain reasonable amounts in sufficient purity in a reproducible way. Batch-to-batch variation also compromises standardization when in vitro citrullination is used to generate the antigen. Insufficient purity of the antigen lowers the specificity of the test because reactivities directed to other components (e.g. the PAD enzyme, the non-citrullinated part of the antigen, other contaminants) may be detected as well. The use of proper (non-citrullinated) controls is therefore very important, as indeed has been noted by Vittecoq and colleagues [16]. Interestingly, autoantigenicity of the PAD protein has recently been described, but this reactivity is not specific for RA [17]. However, despite these limitations several studies have successfully improved the sensitivity of AFA detection while maintaining specificity levels [18]. Using various technologies, assays with various sources of filaggrin have been developed, allowing sensitivities up to 60% [16, 19, 20]. Using citrullinated fibrinogen, Nielen and colleagues [21] reported a similar sensitivity (56%) for a cohort of early arthritis patients.
The use of synthetic citrullinated peptides for anti-citrullinated protein antibody detection can overcome many of the complicating factors of the protein-based approach. Synthetic peptide production and purification is cheap and easily standardized, and via peptides one can synthesize an unlimited pool of defined epitopes. Furthermore, citrulline residues can be incorporated during synthesis of the peptides, leading to a homogeneous preparation of citrullinated molecules. A major breakthrough came with the development of an enzyme-linked immunosorbent assay (ELISA) that used filaggrin-based citrullinated peptides [14]. The reactivity of RA sera was completely dependent on the citrulline residue(s) present, since the same peptides in which the citrulline was replaced by another amino acid were not antigenic. The variation in reactivity patterns against different citrullinated peptides clearly showed that the anti-CCP response in RA is polyclonal. When a filaggrin-based cyclic peptide (cyclization increased the sensitivity) was applied in the first generation anti-CCP (CCP1) test, a sensitivity of 68% was obtained with very high specificity for RA (98%) [22]. Though better than the protein-based methods, the sensitivity was not as high as that of the routinely used RF test. Because filaggrin is not present in the synovium, dedicated libraries of citrulline-containing peptides were screened with RA sera to select for superior epitopes. This culminated in the CCP2 test, which displays a sensitivity of up to 80% without loss of specificity.
Anti-CCP2 autoantibodies as diagnostic markers
Diagnostic markers of disease ideally fulfil three requirements: (i) good sensitivity, to detect a high percentage of patients; (iii) good specificity, to limit false-positive results as much as possible; and (iii) early presence, to facilitate early diagnosis.
Over the last decade many studies have investigated the diagnostic performance of the anti-CCP test. Those using the CCP1 test have been reviewed by van Boekel and colleagues [6]. Increasing data on the improved [23] second-generation anti-CCP test show that the CCP2 test result is a good diagnostic parameter for (early) RA.
Sensitivity/specificity
The first large cohort studies of anti-CCP2 as a diagnostic marker showed that anti-CCP2 combines RF-like sensitivity with almost absolute specificity for RA [5, 24, 25]. These multicentre studies showed that anti-CCP2 antibodies, just like RF, are present in about 80% of established RA patients. In the healthy control group and the non-RA disease controls, the CCP2 test was only positive in maximally 1 and 5%, respectively. The corresponding percentages of the RF (over 10% of healthy controls and more than 20% of disease controls) were markedly higher [5, 25]. Several recent independent studies confirmed these sensitivity/specificity data for CCP2 [26–30]. Using a cohort of 549 RA patients, the study of Suzuki and colleagues [26] clearly showed higher discriminative ability for the CCP2 test than for RF test. The reported sensitivity of 65% (at 96% specificity) by Dubucquoi and colleagues [27] was found in a group of RA patients that included several patients with recent onset of disease. The sensitivity in their established RA patient group was 77%. Overall, the observed intercohort variations in these studies might be explained by differences in the characteristics of the patients that were included. One study reported a somewhat lower specificity for RA of about 90% with the CCP2 test [31], but this could have been caused by the fact that the juvenile RA (JRA) group included in this study might have contained several adult RA patients with an early onset of disease, as discussed previously [32]. The JRA patients had a high average age (31 yr) and often longstanding disease (21 yr on average), mostly resulting in erosions (87%). A striking example of the diagnostic performance of the CCP2 test is the recent study by de Rycke and colleagues [28]. Setting the specificity values for RA at 98.5% resulted in a sensitivity for RF of only 12.8% compared with 73.7% for anti-CCP2.
Taken together, these studies show that the anti-CCP2 test at least equals the RF level for sensitivity, but combines this with far better specificity. The fact that around 40% of RF-seronegative patients appear to be anti-CCP-positive substantiates the additional diagnostic potential of CCP [29, 33].
The anti-CCP test also enables clinicians to effectively distinguish RA patients from other arthritic diseases in cases where the RF is not always discriminative. One of the first examples of such a role in differential diagnosis comes from patients with erosive systemic lupus erythematosus (SLE). Mediwake and colleagues [34] showed that anti-CCP (in this case CCP1) can be used to distinguish RA patients from SLE patients who present with erosive polyarthritis, which is often accompanied by RF seropositivity. Another disease that can readily be misdiagnosed because it often reveals RA-like arthropathies is chronic hepatitis C virus (HCV) infection, which is often accompanied by a positive RF. Wener et al. [35] reported a good discriminative ability of anti-CCP2 over RF in a group of randomly selected HCV patients (44% RF+, none CCP2+). These data were confirmed by Bombardieri and colleagues [36]. Whereas RF was detected in 15% of the HCV patients (37% in case of joint involvement), no anti-CCP2 positivity was seen in these patients. The value of anti-CCP for use in differential diagnosis was also shown when comparing RA patients with polymyalgia rheumatica patients [37]. Taken together, these data clearly outline the diagnostic strength of the anti-CCP test for RA.
Recently, some papers have reported anti-CCP positivity in arthritic diseases that share certain features with RA [38–40]. In palindromic arthritis (PR), a percentage of anti-CCP positivity (56%) similar to that of patients with early RA (55%) was found [38]. PR is a relapsing from of arthritis that shares many features of RA. In addition to the observed anti-CCP reactivity, many of these sera are also RF-positive (30–60%). Moreover, as is the case for RA, PR is associated with the presence of the MHC shared epitope (odds ratio of 2.9) [41]. The current assumption is that PR is an abortive form of RA that in a percentage of patients may develop into RA. The presence of RF in PR has already been shown to be associated with the development of chronic disease [42]. Regarding the prognostic significance of anti-CCP positivity in PR and the possible development of RA, additional studies with larger cohorts and longer follow-up are needed.
In patients with primary Sjögren's syndrome, anti-CCP reactivity was detectable in 8% of the 134 patients tested [39]. For another RA-like disease, psoriatic arthritis, the group of de Rycke [40] found anti-CCP positivity in 8% of the 192 patients tested. This reactivity, confirmed with an additional test using home-made citrullinated peptides, was higher than could be ascribed to simple overlap with RA. These studies make clear that, especially in RA-like arthritic disease entities, further clinical follow-up is needed to establish whether the anti-CCP positivity predicts the development of RA.
Early presence and predictive potential
Because RA patients at their first visit to the clinician often do not fulfil the criteria for the diagnosis/classification of RA, an early detectable, highly predictive marker would greatly help the clinician in reaching a diagnosis. Obviously, the sensitivity and specificity of such a marker should be as high as possible. Recently, two studies, both making use of dated samples from RA patients who were former blood donors, reported the presence of anti-CCP antibodies prior to the appearance of the first clinical symptoms of arthritis [43, 44]. Samples from 72 blood donors were characterized by Nielen and colleagues [43] for positivity of IgM-RF or anti-CCP1. Both serological markers were detectable long before the disease became clinically overt. In some patients, anti-CCP1 was found up to 14 yr prior to the first clinical symptoms of disease. The same was found for 39% of the patients 5.3 yr (median) before the first visit to the clinic. IgM-RF was also found in predisease samples, but not as far back (up to 10 yr) and in a smaller percentage of patients (23% at a median of 3.3 yr). The second study, with a similar set-up, detected anti-CCP2 and RF up to 10 yr before clinical disease in predisease blood samples of 83 RA patients. Anti-CCP2 positivity gradually increased in the years prior to the first clinical symptoms and had reached a positivity of 70% at the time the patients visited the rheumatology clinic for the first time. The sensitivity for detecting RF autoantibodies in these predisease samples was slightly less than for anti-CCP2 [44]. From these studies it is clear that the production of anti-CCP and RF autoantibodies is an early process in RA development, and that their presence is predictive for the development of this disease.
Recent data from several longitudinal studies confirm the predictive ability of anti-CCP2 for RA development [16, 45, 46]. van Gaalen and colleagues [45] used serological markers to predict which of the patients attending an early arthritis clinic, who were classified as undifferentiated arthritis (UA), would progress to RA within the next few years. Follow-up data of 318 patients with UA clearly showed the predictive potential of anti-CCP autoantibodies for the development of RA. After 1 yr of follow-up, 75% of the UA patients who were anti-CCP2-positive at baseline had already progressed to RA. This percentage increased to 93% after 3 yr (odds ratio 38). Of the UA group who were anti-CCP2 negative at baseline, only 25% were classified RA after 3 yr. Similar results were reported by Vittecoq and colleagues [16]. In their cohort of 314 early arthritis patients, 90% of the anti-CCP2-positive patients were classified as RA patients at the 1-yr follow-up. Thus, the combined early presence and predictive ability of anti-CCP may find an important clinical application in the design of treatment strategies.
Anti-CCP2 autoantibodies as a prognostic marker
Various studies have addressed the prognostic value of anti-CCP antibodies. Though the anti-CCP test has only recently become widely available, several studies have already demonstrated its ability to predict the erosiveness of developing RA. Most of these studies used the CCP1 test and the results of these have been discussed previously [32, 47]. An increasing number of studies with anti-CCP2 confirm the prognostic potential. First, in a cohort of 379 early RA patients, Forslind and colleagues [48] showed that anti-CCP2 positivity at baseline (55%) predicts radiological damage and progression at 2 yr follow-up. Similar results were obtained by Kastbom and colleagues [49]. In their study, CCP2 positivity at baseline predicted disease activity at the 3-yr follow-up. In addition, Rönnelid et al. [50] showed that anti-CCP2-positive early RA patients developed worse clinical disease and greater radiological damage within a few years in comparison with anti-CCP2-negative patients.
The prognostic ability of the anti-CCP test is often complemented by other disease parameters. With respect to the presence of the shared epitope MHC class II molecules, this was reported by independent studies of Berglin et al. [51], van Gaalen et al. [52], de Rycke et al. [28] and Lindqvist et al. [53]. The study by de Rycke and colleagues also showed that anti-CCP2 positivity, unlike RF, did not correlate with the presence of extra-articular manifestations [28]. Raza and colleagues [54] reported that the combination of anti-CCP2 and RF positivity was the best prognosticator for the development of persisting RA in a patient group with very early synovitis. Anti-CCP2 levels are also associated with the development of bone erosions in RA [55]. On the other hand, Gossec et al. [56] reported that there was no significant correlation between the absence of anti-CCP antibodies and remission of early RA.
The conclusion from these studies is that a positive anti-CCP test appears to predict the development of erosive RA and that this predictive value complements that of RF. Together with additional individual data (e.g. the presence of shared epitope, family members with RA), this may lead to a very high probability that erosive RA is developing.
Effect of therapy on anti-CCP status
Among the most effective drugs for the treatment of RA at present are those targeting the pro-inflammatory TNF-α. TNF-α-inhibiting agents like infliximab can reduce disease activity and delay radiographic progression of RA [57]. However, side-effects, such as an increased risk of infections and the induction of autoantibodies (e.g. antinuclear antibodies or anti-double-stranded DNA antibodies), have also been reported [58]. The determination of autoantibody levels during the course of treatment (anti-CCP, RF) may give some clues regarding the effectiveness of the treatment and the role of these antibodies in the disease process.
In the past year, several papers have reported effects on RA-associated markers, including anti-CCP, in patients treated with disease-modifying anti-rheumatic drugs (DMARDs) [mainly methotrexate (MTX)] with [59–63] or without [50, 64] infliximab (summarized in Table 1). Bobbio-Pallavicini and colleagues [59] studied autoantibody profiles during long-term (78 weeks) combination treatment with infliximab and MTX. Though treatment resulted in a significant decrease in disease activity scores, no changes in the percentages of patients who were positive for anti-CCP2 or IgM-RF were observed. Titres of RF, on the other hand, were significantly reduced, unlike those of anti-CCP. These results were confirmed by several other studies, although follow-up periods varied between the individual studies (Table 1). In the study of Nissinen and colleagues [60], anti-CCP (measured by the anti-CCP1 test) did not change during 6 weeks of follow-up, though 60% of the patients had a significant clinical response. IgM-RF levels were somewhat decreased in the first weeks of therapy. With follow-up periods of 22 and 30 weeks, respectively, Caramaschi and colleagues [63] and de Rycke and colleagues [62] also observed a decrease in RF levels but not of anti-CCP. In contrast to the previous studies, Alessandri and colleagues [61] did find a small but significant decrease in anti-CCP levels at week 24 of treatment in the patients with clinical improvement. This effect, also observed for RF, was dependent on infliximab, since reductions in anti-CCP levels were not observed after treatment with MTX alone. Nevertheless, DMARD-only therapy can result in a significant (>25%) reduction in both anti-CCP and RF in about 50% of patients [64]. In this last study, effective treatment correlated with a reduction of RF. Recently, Rönnelid and colleagues [50] showed that treatment with sulphasalazine, but not other DMARDs, resulted in a drop in anti-CCP levels, but this decrease occurred only in the first year of follow-up and did not correlate with clinical indicators.
Features of longitudinal studies on anti-CCP status during RA treatment
| Study . | Reference . | Treatment . | Cohort . | Follow-up . | Outcome . |
|---|---|---|---|---|---|
| Bobbio-Pallavicini | [59] | Infliximab/MTX | 39 | 78 weeks | No effect on anti-CCP levels (87% responders) |
| Nissinen | [60] | Infliximab/DMARDs | 25 | 6 weeks | No effect on anti-CCP levels (60% responders) |
| Caramaschi | [63] | Infliximab/MTX | 27 | 22 weeks | No effect on anti-CCP levels (74% responders) |
| de Rycke | [62] | Infliximab/MTX | 62 | 30 weeks | No effect on anti-CCP levels (100% responders) |
| Alessandri | [61] | Infliximab/MTX | 43 | 24 weeks | Decrease in anti-CCP in patients with clinical improvement |
| Mikuls | [64] | DMARDs | 208 | 2 yr | Small anti-CCP reduction in 50% of patients; no association with response |
| Rönnelid | [50] | DMARDs | 379 | 5 yr | Anti-CCP stable; only small drop in 1st year (not correlating with disease) |
| Study . | Reference . | Treatment . | Cohort . | Follow-up . | Outcome . |
|---|---|---|---|---|---|
| Bobbio-Pallavicini | [59] | Infliximab/MTX | 39 | 78 weeks | No effect on anti-CCP levels (87% responders) |
| Nissinen | [60] | Infliximab/DMARDs | 25 | 6 weeks | No effect on anti-CCP levels (60% responders) |
| Caramaschi | [63] | Infliximab/MTX | 27 | 22 weeks | No effect on anti-CCP levels (74% responders) |
| de Rycke | [62] | Infliximab/MTX | 62 | 30 weeks | No effect on anti-CCP levels (100% responders) |
| Alessandri | [61] | Infliximab/MTX | 43 | 24 weeks | Decrease in anti-CCP in patients with clinical improvement |
| Mikuls | [64] | DMARDs | 208 | 2 yr | Small anti-CCP reduction in 50% of patients; no association with response |
| Rönnelid | [50] | DMARDs | 379 | 5 yr | Anti-CCP stable; only small drop in 1st year (not correlating with disease) |
Features of longitudinal studies on anti-CCP status during RA treatment
| Study . | Reference . | Treatment . | Cohort . | Follow-up . | Outcome . |
|---|---|---|---|---|---|
| Bobbio-Pallavicini | [59] | Infliximab/MTX | 39 | 78 weeks | No effect on anti-CCP levels (87% responders) |
| Nissinen | [60] | Infliximab/DMARDs | 25 | 6 weeks | No effect on anti-CCP levels (60% responders) |
| Caramaschi | [63] | Infliximab/MTX | 27 | 22 weeks | No effect on anti-CCP levels (74% responders) |
| de Rycke | [62] | Infliximab/MTX | 62 | 30 weeks | No effect on anti-CCP levels (100% responders) |
| Alessandri | [61] | Infliximab/MTX | 43 | 24 weeks | Decrease in anti-CCP in patients with clinical improvement |
| Mikuls | [64] | DMARDs | 208 | 2 yr | Small anti-CCP reduction in 50% of patients; no association with response |
| Rönnelid | [50] | DMARDs | 379 | 5 yr | Anti-CCP stable; only small drop in 1st year (not correlating with disease) |
| Study . | Reference . | Treatment . | Cohort . | Follow-up . | Outcome . |
|---|---|---|---|---|---|
| Bobbio-Pallavicini | [59] | Infliximab/MTX | 39 | 78 weeks | No effect on anti-CCP levels (87% responders) |
| Nissinen | [60] | Infliximab/DMARDs | 25 | 6 weeks | No effect on anti-CCP levels (60% responders) |
| Caramaschi | [63] | Infliximab/MTX | 27 | 22 weeks | No effect on anti-CCP levels (74% responders) |
| de Rycke | [62] | Infliximab/MTX | 62 | 30 weeks | No effect on anti-CCP levels (100% responders) |
| Alessandri | [61] | Infliximab/MTX | 43 | 24 weeks | Decrease in anti-CCP in patients with clinical improvement |
| Mikuls | [64] | DMARDs | 208 | 2 yr | Small anti-CCP reduction in 50% of patients; no association with response |
| Rönnelid | [50] | DMARDs | 379 | 5 yr | Anti-CCP stable; only small drop in 1st year (not correlating with disease) |
From these studies, it is clear that the anti-CCP autoantibody system is different from the RF system with respect to response to treatment. Clearly, investigating the functional importance of anti-CCP in the progression of RA demands other approaches specifically aimed at decreasing this humoral response.
Putative functional implications for anti-CCP
Because anti-CCP autoantibodies are so specific for RA, one wonders what they can tell us about the aetiology of the disease. To answer that question we need to know more about (synovial) protein citrullination and the PAD enzymes responsible for the conversion of peptidylarginine to peptidylcitrulline. The five isoforms of human PAD show a tissue-specific expression profile [9]. It has been shown that PAD enzymes are present in the inflamed synovium and that their activity is regulated at the transcriptional and translational levels [65, 66]. In addition, these enzymes require relatively high Ca2+ concentrations, about 100 times higher than normally present in the cytosol of a living cell. Interestingly, citrullination occurs primarily in dying cells. Indeed, during inflammation, when many cells die by apoptosis or necrosis, one can detect citrullinated proteins, both in animal models of inflammation and in the inflamed synovia of RA and non-RA patients. Paradoxically, the presence of citrullinated proteins in most cases does not lead to the generation of anti-citrullinated protein antibodies [67, 68]. This phenomenon might be related to the genetic background of the patient. It has been known for some time that there is a rather strong correlation between RA and certain HLA-DR alleles, particularly HLA-DRB1*0401 and HLA-DRB1*0404. These alleles contain the so-called shared epitope (SE) motif. Hill and colleagues [69] showed that the conversion of arginine to citrulline increased the affinity of a peptide for binding to HLA-DRB1 and can lead to activation of CD4+ T cells in DR4-transgenic mice. These results thus indicate that the production of anti-citrullinated protein antibodies is dependent on the presence of certain susceptibility genes for RA. The fact that the observed odds ratio of 66.8 for the combination of anti-CCP and SE HLA gene carriage is over two times the value of the multiplication of the corresponding single odds ratios (anti-CCP, 15.9; SE, 2.4) also suggests that these factors are functionally associated [51]. Very recently it was shown that patients who were homozygous for SE alleles and seropositive for anti-CCP antibody presented with an increased rate of joint destruction compared with patients carrying only one or none of these alleles [52]. Also, these results suggest that the production of these antibodies stimulates the ongoing inflammation in RA.
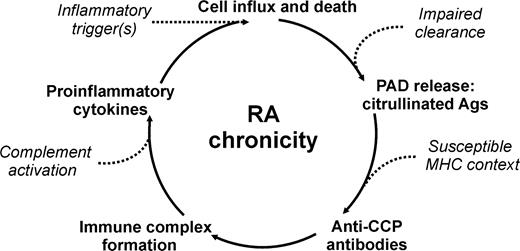
These data have led to a relatively simple model for the development of RA, depicted in Fig. 1 and discussed previously [70, 71]. In individuals with a genetic predisposition for RA, an inflammation of the joint, in itself innocent, leads to infiltration of inflammatory cells (monocytes, granulocytes) that contain PAD enzymes. After activation, these cells will die via apoptosis and will be cleared (removed) by phagocytosing cells. However, when there is massive apoptosis, or a (genetic) defect in the clearance system, some apoptotic cells may become necrotic, thereby releasing citrullinated proteins (histones, vimentin) and PAD enzymes. The PAD enzymes can subsequently citrullinate synovial proteins, such as fibrin. In 99% of individuals this will be the end of the story. In the 1% of individuals who are able to present fragments of the citrullinated proteins to the T cells via certain MHC class II molecules, a B-cell response to citrullinated antigens will be generated, resulting in the formation of immune complexes which will stimulate the inflammatory process by upregulation of pro-inflammatory cytokines. New monocytes and granulocytes will enter the synovium, will be activated, die, and stimulate a new flare of inflammation. The repetition of this process over a number of years, eventually accompanied by fresh traumas or environmental events that stimulate inflammation, will finally lead to a chronic inflammation which can develop into the disease we know as rheumatoid arthritis. In this way, anti-CCP contributes to the perpetuation of joint inflammation and thereby to the chronicity and severity of RA.
Future perspectives
Currently available therapies for RA, nicely reviewed by Smolen and Steiner [72], are mainly anti-inflammatory and unable to cure the disease. At best these therapies are able to slow down the extent of swelling and erosive damage. More insight obtained over recent years suggests that combination therapies given early in the disease have the greatest therapeutic potential [73]. To achieve the ultimate goal of curative treatment, it is crucial to identify RA patients before joint damage occurs. The fact that the CCP2 test is now widely available will speed up these studies, as shown by the burst of data on CCP in the literature. Knowledge about the citrullinome (total repertoire of citrullinated proteins), the application of array-based screening technologies [74] and use in automated assays [75] will undoubtedly further increase the diagnostic potential of these autoantibodies.
A.Z. received a grant from the Netherlands Organisation for Scientific Research. G.P. and W.V. are cofounders and shareholders of ModiQuest b.v. W.V. serves as a consultant to Euro-Diagnostica b.v. and Axis shield.
References
Arnett FC, Edworthy SM, Bloch DA et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis.
Visser H, le Cessie S, Vos K et al. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis.
Mageed RA. The RF antigen. In: Smolen JS, Kalden JR, Maini RN, eds.
van Venrooij WJ, Hazes JM, Visser H. Anticitrullinated protein/peptide antibody and its role in the diagnosis and prognosis of early rheumatoid arthritis.
van Boekel MA, Vossenaar ER, van den Hoogen FH, van Venrooij WJ. Autoantibody systems in rheumatoid arthritis: specificity, sensitivity and diagnostic value.
Steiner G, Smolen JS. Autoantibodies in rheumatoid arthritis and their clinical significance.
Marcelletti JF, Nakamura RM. Assessment of serological markers associated with rheumatoid arthritis: diagnostic autoantibodies and conventional disease activity markers.
Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease.
Nienhuis RL, Mandema E. A new serum factor in patients with rheumatoid arthritis; the antiperinuclear factor (APF).
Young BJ, Mallya RK, Leslie RD et al. Anti-keratin antibodies in rheumatoid arthritis. In: van Venrooij WJ, Maini RN, eds.
Hoet RM, van Venrooij WJ.
Sebbag M, Simon M, Vincent C et al. The antiperinuclear factor and the so-called antikeratin antibodies are the same rheumatoid arthritis-specific autoantibodies.
Schellekens GA, de Jong BA, van den Hoogen FH et al. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies.
Girbal-Neuhauser E, Durieux JJ, Arnaud M et al. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues.
Vittecoq O, Incaurgarat B, Jouen-Beades F et al. Autoantibodies recognizing citrullinated rat filaggrin in an ELISA using citrullinated and non-citrullinated recombinant proteins as antigens are highly diagnostic for rheumatoid arthritis.
Nissinen R, Paimela L, Julkunen H et al. Peptidylarginine deiminase, the arginine to citrulline converting enzyme, is frequently recognized by sera of patients with rheumatoid arthritis, systemic lupus erythematosus and primary Sjogren syndrome.
Nogueira L, Sebbag M, Vincent C et al. Performance of two ELISAs for antifilaggrin autoantibodies, using either affinity purified or deiminated recombinant human filaggrin, in the diagnosis of rheumatoid arthritis.
Vincent C, Nogueira L, Sebbag M et al. Detection of antibodies to deiminated recombinant rat filaggrin by enzyme-linked immunosorbent assay: a highly effective test for the diagnosis of rheumatoid arthritis.
Union A, Meheus L, Humbel RL et al. Identification of citrullinated rheumatoid arthritis-specific epitopes in natural filaggrin relevant for antifilaggrin autoantibody detection by line immunoassay.
Nielen MM, van der Horst AR, van Schaardenburg D et al. Antibodies to citrullinated human fibrinogen (ACF) have diagnostic and prognostic value in early arthritis.
Schellekens GA, Visser H, de Jong BA et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide.
van Gaalen FA, Visser H, Huizinga TW. A comparison of the diagnostic accuracy and prognostic value of the first- and second anti-cyclic citrullinated peptides autoantibody (CCP1 and CCP2) tests for rheumatoid arthritis.
Pinheiro GC, Scheinberg MA, Aparecida da Silva M, Maciel S. Anti-cyclic citrullinated peptide antibodies in advanced rheumatoid arthritis.
Vasishta A. Diagnosing early-onset rheumatoid arthritis: the role of anti-CCP antibodies.
Suzuki K, Sawada T, Murakami A et al. High diagnostic performance of ELISA detection of antibodies to citrullinated antigens in rheumatoid arthritis.
Dubucquoi S, Solau-Gervais E, Lefranc D et al. Evaluation of anti-citrullinated filaggrin antibodies as hallmarks for the diagnosis of rheumatic diseases.
de Rycke L, Peene I, Hoffman IE et al. Rheumatoid factor and anti-citrullinated protein antibodies in rheumatoid arthritis: diagnostic value, associations with radiological progression rate, and extra-articular manifestations.
Vallbracht I, Rieber J, Oppermann M et al. Diagnostic and clinical value of anti-cyclic citrullinated peptide antibodies compared with rheumatoid factor isotypes in rheumatoid arthritis.
Grootenboer-Mignot S, Nicaise-Roland P, Delaunay C et al. Second generation anti-cyclic citrullinated peptide (anti-CCP2) antibodies can replace other anti-filaggrin antibodies and improve rheumatoid arthritis diagnosis.
Lee DM, Schur PH. Clinical utility of the anti-CCP assay in patients with rheumatic diseases.
Vossenaar ER, van Venrooij WJ. Anti-CCP antibodies, a highly specific marker for (early) rheumatoid arthritis.
Kroot EJ, de Jong BA, van Leeuwen MA et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis.
Mediwake R, Isenberg DA, Schellekens GA, van Venrooij WJ. Use of anti-citrullinated peptide and anti-RA33 antibodies in distinguishing erosive arthritis in patients with systemic lupus erythematosus and rheumatoid arthritis.
Wener MH, Hutchinson K, Morishima C, Gretch DR. Absence of antibodies to cyclic citrullinated peptide in sera of patients with hepatitis C virus infection and cryoglobulinemia.
Bombardieri M, Alessandri C, Labbadia G et al. Role of anti-cyclic citrullinated peptide antibodies in discriminating patients with rheumatoid arthritis from patients with chronic hepatitis C infection-associated polyarticular involvement.
Lopez-Hoyos M, Ruiz DA, Blanco R et al. Clinical utility of anti-CCP antibodies in the differential diagnosis of elderly-onset rheumatoid arthritis and polymyalgia rheumatica.
Salvador G, Gomez A, Vinas O et al. Prevalence and clinical significance of anti-cyclic citrullinated peptide and antikeratin antibodies in palindromic rheumatism. An abortive form of rheumatoid arthritis?
Gottenberg JE, Mignot S, Nicaise-Rolland P et al. Prevalence of anti-cyclic citrullinated peptide and anti- keratin antibodies in patients with primary Sjogren's syndrome.
Vander Cruyssen B, Hoffman IE, Zmierczak H et al. Anti-citrullinated peptide antibodies may occur in patients with psoriatic arthritis.
Sanmarti R, Canete JD, Salvador G. Palindromic rheumatism and other relapsing arthritis.
Gonzalez-Lopez L, Gamez-Nava JI, Jhangri GS et al. Prognostic factors for the development of rheumatoid arthritis and other connective tissue diseases in patients with palindromic rheumatism.
Nielen MM, van Schaardenburg D, Reesink HW et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors.
Rantapää-Dahlqvist S, de Jong BA, Berglin E et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis.
van Gaalen FA, Linn-Rasker SP, van Venrooij WJ et al. Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study.
Soderlin MK, Kastbom A, Kautiainen H et al. Antibodies against cyclic citrullinated peptide (CCP) and levels of cartilage oligomeric matrix protein (COMP) in very early arthritis: relation to diagnosis and disease activity.
Zendman AJ, Vossenaar ER, van Venrooij WJ. Autoantibodies to citrullinated (poly)peptides: a key diagnostic and prognostic marker for rheumatoid arthritis.
Forslind K, Ahlmén M, Eberhardt K et al. Prediction of radiological outcome in early RA in clinical practice: role of antibodies to citrullinated peptides (anti-CCP).
Kastbom A, Strandberg G, Lindroos A, Skogh T. Anti-CCP antibody test predicts the disease course during three years in early rheumatoid arthritis (the TIRA project).
Rönnelid J, Wick MC, Lampa J et al. Longitudinal analysis of anti-citrullinated protein/peptide antibodies (anti-CP) during 5 year follow-up in early rheumatoid arthritis: anti-CP status is a stable phenotype that predicts worse disease activity and greater radiological progression.
Berglin E, Padyukov L, Sundin E et al. A combination of autoantibodies to cyclic citrullinated peptide (CCP) and HLA-DRB1 locus antigens is strongly associated with future onset of rheumatoid arthritis.
van Gaalen FA, van Aken J, Huizinga TW et al. Association between HLA class II genes and autoantibodies to cyclic citrullinated peptides (CCPs) influences the severity of rheumatoid arthritis.
Lindqvist E, Eberhardt K, Bendtzen K et al. Prognostic laboratory markers of joint damage in rheumatoid arthritis.
Raza K, Breese M, Nightingale P et al. Predictive value of antibodies to cyclic citrullinated peptide in patients with very early inflammatory arthritis.
Maddali-Bongi S, Manetti R, Melchiorre D et al. Anti-cyclic citrullinated peptide antibodies are highly associated with severe bone lesions in rheumatoid arthritis anti-CCP and bone damage in RA.
Gossec L, Dougados M, Goupille P et al. Prognostic factors for remission in early rheumatoid arthritis: a multiparameter prospective study.
Lipsky PE, van der Heijde DM, St Clair EW et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group.
Maini RN, St Clair EW, Breedveld F et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group.
Bobbio-Pallavicini F, Alpini C, Caporali R et al. Autoantibody profile in rheumatoid arthritis during long-term infliximab treatment.
Nissinen R, Leirisalo-Repo M, Peltomaa R et al. Cytokine and chemokine receptor profile of peripheral blood mononuclear cells during treatment with infliximab in patients with active rheumatoid arthritis.
Alessandri C, Bombardieri M, Papa N et al. Decrease of anti-cyclic citrullinated peptide antibodies and rheumatoid factor following anti-TNFalpha therapy (infliximab) in rheumatoid arthritis is associated with clinical improvement.
de Rycke L, Verhelst X, Kruithof E et al. Rheumatoid factor, but not anti-citrullinated protein antibodies, is modulated by infliximab treatment in rheumatoid arthritis.
Caramaschi P, Biasi D, Tonolli E et al. Antibodies against cyclic citrullinated peptides in patients affected by rheumatoid arthritis before and after infliximab treatment.
Mikuls TR, O’Dell JR, Stoner JA et al. Association of rheumatoid arthritis treatment response and disease duration with declines in serum levels of IgM rheumatoid factor and anti-cyclic citrullinated peptide antibody.
Chapuy-Regaud S, Sebbag M, Nachat R et al. Peptidylarginine deiminase isoforms expressed in the synovial membrane of rheumatoid arthritis patients.
Vossenaar ER, Radstake TR, van der Heijden A et al. Expression and activity of citrullinating PAD enzymes in monocytes and macrophages.
Vossenaar ER, Smeets TJ, Kraan MC et al. The presence of citrullinated proteins is not specific for rheumatoid synovial tissue.
Chapuy-Regaud S, Sebbag M, Baeten D et al. Fibrin deimination in synovial tissue is not specific for rheumatoid arthritis but commonly occurs during synovitides.
Hill JA, Southwood S, Sette A et al. The conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule.
Vossenaar ER, Zendman AJ, van Venrooij WJ. Citrullination, a possible functional link between susceptibility genes and rheumatoid arthritis.
Vossenaar ER, van Venrooij WJ. Citrullinated proteins: sparks that may ignite the fire in rheumatoid arthritis.
Smolen JS, Steiner G. Therapeutic strategies for rheumatoid arthritis.
O’Dell JR. Treating rheumatoid arthritis early: a window of opportunity?
Robinson WH, DiGennaro C, Hueber W et al. Autoantigen microarrays for multiplex characterization of autoantibody responses.




Comments