-
PDF
- Split View
-
Views
-
Cite
Cite
Y. Asano, H. Ihn, N. Asashima, N. Yazawa, Y. Mimura, M. Jinnin, K. Yamane, K. Tamaki, A case of diffuse scleroderma successfully treated with high-dose intravenous immune globulin infusion, Rheumatology, Volume 44, Issue 6, June 2005, Pages 824–826, https://doi.org/10.1093/rheumatology/keh600
Close - Share Icon Share
Sir, A 60-yr-old Japanese female visited our hospital in August of 2000 complaining of Raynaud's phenomenon. On physical examination, skin sclerosis was evident on the fingers, hands, forearms, upper arms, face, chest, abdomen, lower legs and the dorsum of the feet. The modified Rodnan total skin thickness score (TSS) [1] was 24. Nailfold bleeding and flexion contractions of the phalanges were also observed. She had no symptoms suggestive of other collagen diseases.
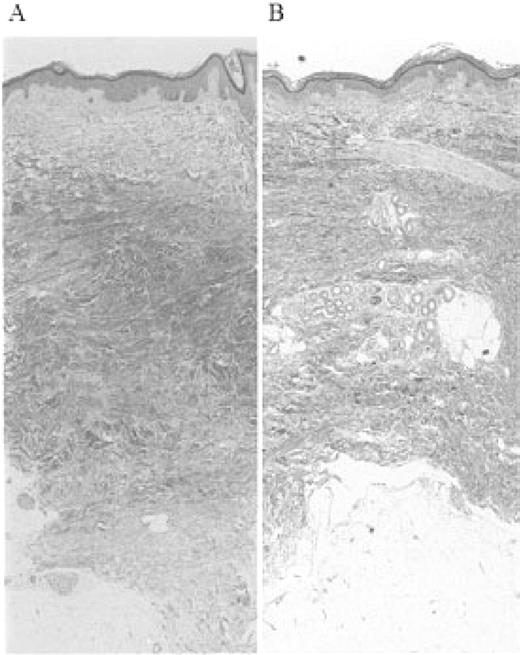
Laboratory examinations revealed an increased erythrocyte sedimentation rate, positivity for antinuclear antibodies (1:320) with a speckled and nucleolar pattern, and a high titre of anti-topoisomerase-I antibody. Though mild pulmonary fibrosis was observed in the bilateral lower lobes by high-resolution CT, pulmonary function tests showed no abnormalities. All other examinations, including blood cell count, serum complement, creatine kinase, urinalysis, electrocardiograph, barium meal and upper gastrointestinal endoscopy, showed normal or negative results. Histology of a skin biopsy specimen obtained from the forearm was typical of scleroderma (Fig. 1A). Based on these findings, she was diagnosed with scleroderma.
Histological analysis of dermal section before and after treatment with IVIG. (A) Before treatment. Biopsy specimen from the dorsum of forearm with thickened collagen bundles and replacement of the subcutaneous fat (haematoxylin–eosin, ×40). (B) After treatment. Biopsy specimen from the dorsum of forearm next to the previous biopsy site, demonstrating loosely arranged collagen fibres and dermal architecture close to normal skin (haematoxylin–eosin, ×40).
She was treated with d-penicillamine. However, she had stable skin sclerosis over 2 yr (TSS changed from 24 to 22). In August of 2002, after stopping taking d-penicillamine for 1 month, she received infusions of high-dose intravenous immunoglobulin (IVIG) at a dose of 400 mg/kg daily for 5 consecutive days. TSS of the patient improved dramatically up to 4 weeks after IVIG (from 22 to 17 at 2 weeks and 14 at 4 weeks), and then gradually improved over 2 yr (from 14 to 7). Histological examination of a skin biopsy specimen (Fig. 1B), which was obtained from the forearm next to the previous biopsy site 4 weeks after IVIG, revealed that the skin thickness was dramatically reduced by IVIG (from 2.8 to 2.0 mm). No adverse effects were observed during or after the treatment.
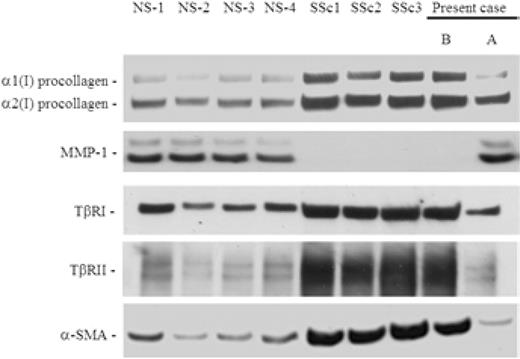
To assess the effect of IVIG on scleroderma fibroblasts, we prepared cultured fibroblasts from skin biopsy samples obtained before and after IVIG as described previously [2]. Using four strains of scleroderma fibroblasts, including the present case, and four strains of normal fibroblasts, which were obtained from control donors matched with each scleroderma patient for age, sex and biopsy site, immunoblotting was performed, as described previously [2] (Fig. 2). The expression of type I procollagen in whole-cell lysates were markedly elevated in scleroderma fibroblasts compared with normal fibroblasts, while the expression of matrix metalloproteinase 1 (MMP-1) in conditioned media were markedly decreased in scleroderma fibroblasts. In the present case, the altered expression of these proteins was restored after IVIG to levels similar to those observed in normal fibroblasts. We next investigated the expression of transforming growth factor β (TGF-β) receptors. Consistent with previous reports [2–4], the expression of TGF-β receptors was markedly elevated in scleroderma fibroblasts. In the present case, the expression of TGF-β receptors was reduced after IVIG to levels similar to those observed in normal fibroblasts. Finally, the expression of α-smooth muscle actin (α-SMA), a marker of myofibroblasts, was determined. The expression of α-SMA was markedly elevated in scleroderma fibroblasts. In the present case, however, the expression of α-SMA was reduced after IVIG to levels similar to those observed in normal fibroblasts. These results indicate that IVIG can restore the altered phenotype of scleroderma fibroblasts.
Effects of IVIG on the expression levels of type I procollagen, MMP-1, TGF-β receptors and α-SMA in scleroderma fibroblasts. Conditioned media were subjected to immunoblotting using anti-MMP-1 antibody. Whole-cell lysates were subjected to immunoblotting using anti-type I collagen antibody, anti-TGF-β receptor type I (TβRI) antibody, anti-TGF-β receptor type II (TβRII) antibody or anti-α-SMA antibody. All bands show one representative of three independent experiments. (B) Before IVIG treatment. (A) After IVIG treatment.
IVIG is a potent immunomodulating agent whose efficacy has been demonstrated in various immune-mediated disorders [5–7]. To date, there is a report of three scleroderma patients whose TSS improved after IVIG therapy [7]. In this study, a patient with diffuse scleroderma had marked improvement in TSS from 2 weeks, and long-term benefit was also confirmed by the follow-up evaluation. Furthermore, the effect of IVIG was confirmed by histological examination.
Previous findings suggest that TGF-β receptors are up-regulated in scleroderma fibroblasts, and this abnormality contributes to the establishment of autocrine TGF-β signalling in those cells [3, 4]. In this study, we demonstrated that the myofibroblastic phenotype of scleroderma fibroblasts was almost completely restored by IVIG. In addition, this observation was accompanied by a reduction in the expression of TGF-β receptors. Taken together, these results suggest that the up-regulated expression of TGF-β receptors is mediated by IVIG-sensitive processes.
To date, several mechanisms have been proposed for the immunomodulatory effect of IVIG [8]: inhibition of complement activities, neutralization of autoantibodies by anti-idiotypic antibodies, modulation of lymphocyte functions and cytokine synthesis, and blockade of Fcγ receptors on phagocytic cells. The present observations suggest that several of these mechanisms may work together in the initiation and maintenance of fibroblast activation in scleroderma.
In summary, we report a case of diffuse scleroderma successfully treated with IVIG. Although additional studies are needed because many treatments, including immunosuppressives, d-penicillamine and minocycline, have been shown to be of benefit in scleroderma only to fail when further investigated, the evidence that IVIG almost completely restores the altered phenotype of scleroderma fibroblasts provides a potential pathophysiological basis for the usefulness of this therapy in scleroderma.
The authors have declared no conflicts of interest.
References
Clements PJ, Lachenbruch PA, Seibold JR et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies.
Asano Y, Ihn H, Yamane K, Kubo M, Tamaki K. Impaired Smad7-Smurf-mediated negative regulation of transforming growth factor (TGF)-β signaling in scleroderma fibroblasts.
Kawakami T, Ihn H, Xu W, Smith E, LeRoy C, Trojanowska M. Increased expression of TGF-β receptors by scleroderma fibroblasts: evidence for contribution of autocrine TGF-β signaling to scleroderma phenotype.
Ihn H, Yamane K, Kubo M, Tamaki K. Blockade of endogenous transforming growth factor-β signaling prevents up-regulated collagen synthesis in scleroderma fibroblasts. Association with increased expression of transforming growth factor-β receptors.
Boletis JN, Aloannidis JP, Boki KA, Moutsopoulos HM. Intravenous immunoglobulin compared with cyclophosphamide for proliferative lupus nephritis.
Cherin P, Herson S, Wechsler B et al. Intravenous immunoglobulin for polymyositis and dermatomyositis.
Levy Y, Sherer Y, Langevitz P et al. Skin score decrease in systemic sclerosis patients treated with intravenous immunoglobulin – a preliminary report.




Comments