-
PDF
- Split View
-
Views
-
Cite
Cite
Mark H. Williams, Clive E. Handler, Raza Akram, Colette J. Smith, Clare Das, Joanna Smee, Devaki Nair, Christopher P. Denton, Carol M. Black, John G. Coghlan, Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertension, European Heart Journal, Volume 27, Issue 12, June 2006, Pages 1485–1494, https://doi.org/10.1093/eurheartj/ehi891
Close - Share Icon Share
Abstract
Aims The aims of this study were to evaluate the diagnostic value and to explore the prognostic value of N-terminal brain natriuretic peptide (N-TproBNP) in patients with systemic sclerosis (SSc) both with and without pulmonary arterial hypertension (PAH).
Methods and results N-TproBNP, six-minute walk distance (SMWD), haemodynamics (at right heart catheterization) or tricuspid gradient (by echocardiography), and survival were assessed in 109 patients with SSc. The study population included 68 individuals with PAH [mean pulmonary artery pressure (PAP) >25 mmHg and pulmonary capillary wedge pressure <15 mmHg] and 41 individuals without PAH. In patients with PAH, the prognostic value of baseline and change in WHO functional class, N-TproBNP levels, and SMWD were compared using Kaplan–Meier survival curves and Cox proportional hazard analysis. The mean duration of follow-up was 10 months (range 1–18 months). One year survival in patients with normal PAP was 100% when compared with 83.5% in those with SSc-PAH (P<0.05). The patients without PAH had a mean N-TproBNP level of 139 pg/mL (SD 151); those with SSc-PAH had a significantly higher mean N-TproBNP level of 1474 pg/mL (SD 2642) (P=0.0002). Among patients with PAH for every order of magnitude increase in N-TproBNP level there was a four-fold increased risk of death (P=0.002 for baseline level and P=0.006 for follow-up level). Baseline N-TproBNP levels were correlated positively with mean PAP (r=0.62; P<0.0001), pulmonary vascular resistance (PVR) (r=0.81; P<0.0001), and inversely with SMWD (r=−0.46; P<0.0001). Among patients with SSc-PAH, 13 patients (19%) were in WHO functional classes II and had mean N-TproBNP levels of 325 pg/mL (SD 388). Fifty-three patients (78%) were in WHO classes III and IV and had significantly higher mean N-TproBNP levels of 1677 pg/mL (SD 2835) (P=0.02). At an N-TproBNP level of 395 pg/mL, the sensitivity and specificity for predicting the presence of SSc-PAH were 56 and 95% respectively.
Conclusion Raised N-TproBNP levels are directly related to the severity of PAH. In screening programs, SSc patients with an N-TproBNP in excess of 395 pg/mL have a very high probability of having pulmonary hypertension. Baseline and serial changes in N-TproBNP levels are highly predictive of survival. A 10-fold increase in N-TproBNP level on therapy is associated with a greater than three-fold increase in mortality, and may indicate therapeutic failure.
Introduction
Pulmonary arterial hypertension (PAH) affects approximately 12% of patients with systemic sclerosis (SSc) and is associated with a worse prognosis than idiopathic pulmonary arterial hypertension (IPAH).1–3 Breathlessness is common in patients with SSc as the condition may affect the lungs, heart, and musculoskeletal system with, thus scleroderma-associated pulmonary artery hypertension (SSc-PAH) is often diagnosed too late for patients to derive maximum benefit from disease-modifying therapies.
End-stage SSc-PAH may be suspected clinically in a patient with SSc who becomes progressively breathless and who has signs of PAH and right heart failure. An accurate method for early diagnosis of SSc-PAH and identifying patients at high risk is important for management. Current non-invasive screening tests are of limited diagnostic and prognostic value.4
We have shown that the prognosis of patients with SSc-PAH can be improved significantly with disease-modifying therapies (including endothelin antagonists and prostanoids) underpinned by modern approaches to care delivery and efforts to diagnose the condition early.5 Further improvements in morbidity and mortality associated with this condition depend on accurate and early diagnosis of PAH as well as more effective therapies.
Our current practice is to screen all patients with SSc for PAH using pulmonary function tests and echocardiography. PAH is likely in breathless patients, particularly those with a reduced transfer factor who have normal lung volumes. However, lung function tests remain insufficiently sensitive to exclude SSc-PAH. Echocardiography has limited value in PAH except at high levels (tricuspid gradient above 45 mmHg) when it correlates well with mean pulmonary artery pressure (mPAP) at right heart cardiac catheterization. Echocardiographic estimations of PAP at levels below 25 mmHg are useful in excluding PAH.4 As a screening tool echocardiography has many limitations, not least being highly operator-dependent and relatively cumbersome to perform to an optimal standard. There is a pressing need for a simpler screening tool to identify patients with SSc who require cardiac catheretization.
PAH is present if the mPAP is >25 mmHg at rest and >30 mmHg after exercise at cardiac catheterization. This test is not, however, useful as a screening tool.
Treatment of SSc-PAH is presently guided largely by symptom severity. Disease-modifying therapies—endothelin antagonists, prostanoids, and phosphodiesterase-5 inhibitors—are licenced in Europe for patients with severe functional limitation (WHO Class III).6 Given the multiple contributors to dyspnoea in SSc, the value of this parameter in managing PAH is less clear than in patients with IPAH.
Six-minute walk distance (SMWD) has been shown to be an independent predictor of mortality in patients with IPAH,7 but not in SSc-PAH.5 In IPAH change in SMWD is used as a guide to the continuing efficacy of therapy. SMWD in the SSc-PAH population may be influenced by musculoskeletal and psychological factors, and so may not represent a true picture of a patient's clinical state.
Brain natriuretic peptide (BNP) is a peptide hormone released from both right (RV) and left ventricular (LV) myocardial cells in response to increased myocardial pressure and/or volume overload.8 An increase in ventricular wall stretch results in activation of the BNP gene transcription, leading to a release of BNP.9 BNP levels are high in LV systolic and diastolic dysfunction and acute coronary syndromes.10–13 It has been shown to be a useful screening test for congestive cardiac failure, and is a strong predictor of morbidity and mortality.10,14–20
There have been few studies of BNP in conditions affecting the right heart. In small studies, BNP has been shown to be elevated in various forms of PAH including IPAH,21 PAH associated with interstitial lung disease,22 congenital systemic-to-pulmonary shunts,23 chronic obstructive pulmonary disease,24 chronic thrombo-embolic disease,25 and SSc-PAH.26 Nagaya et al.27 studied the relationship between baseline and 3-month BNP levels and survival in 60 patients with IPAH, however, his findings have yet to be confirmed, and the more stable N-TproBNP analogue has not been evaluated in a prognostic study of a sizable population with PAH.
We have previously proposed the use of N-TproBNP as a screening tool for SSc-PAH. This previous study included 49 patients with scleroderma of which 23 patients had PAH confirmed at right heart catheterization and 26 did not have PAH. The mean value of N-TproBNP for patients with SSc-PAH and without PAH were 3365 and 347 pg/mL, respectively. Receiver operating characteristic (ROC) curve analysis showed that a cut-off value of 395 pg/mL had a sensitivity of 69% and a specificity of 100% for SSc-PAH.26
The aims of this larger study were to assess prospectively the specificity of 395 pg/mL in a larger population and to evaluate the prognostic value of N-TproBNP in a homogenous group of patients with SSc-PAH.
Methods
Population
Patients were selected from the population attending the National Pulmonary Hypertension service screening and treatment program at the Royal Free Hospital. Those with SSc-PAH were selected from the 134 patients under active follow-up between August 2003 and April 2004 using the criteria set out below. Those without PAH included patients in whom SSc-PAH was excluded at catheter study for suspected PAH (precise criteria detailed under patient selection) and patients randomly selected from routine follow-up clinics of SSc patients in whom annual screening assessment suggested a very low likelihood of PAH. Recruitment was planned to include a minimum of 60 with SSc-PAH and 40 controls.
Ethical considerations
Local Research and Ethics Committee approval and informed consent was obtained from all subjects for all testing. The study complied with the Declaration of Helsinki.
Patient selection
All patients met the Arthritis and Rheumatism Council criteria for diagnosis of systemic sclerosis.28 Patients were recruited from our twice weekly pulmonary hypertension outpatients clinic using the following criteria:
They had not been involved in our previous study of N-TproBNP levels in SSc-PAH.
Cardiac catheterization had been performed within the previous 6 months or was planned. Patients who declined routine catheterization (n=12) were not excluded, where their condition had remained stable from the previous catheterization.
Patients were excluded if they had conditions known to affect N-TproBNP:
Significant renal impairment (creatinine >150 µmol/L].
Evidence of LV impairment on echocardiography or at cardiac catheterization (pulmonary capillary wedge pressure >15 mmHg).
The control population included all patients who had undergone formal right and left heart catheterization and were found to have normal cardiac and pulmonary vascular findings on left and right heart study. As the numbers of such patients were quite small, this number was augmented using patients from our routine screening service, where the diagnosis of cardiac and pulmonary vascular disease was felt to be rigorously excluded: LV ejection fraction >55%; normal diastolic parameters; systemic blood pressure <140/85 mmHg without therapy; normal renal function; tricuspid gradient <2.5 m/s (25 mmHg); dyspnoea Grade 1 and TLCO >80% of predicted.
Right heart catheterization
Right heart catheterization was performed in accordance with our previous reported protocol.1 A vasodilator trial was not performed as we have found this to provide no useful information on patients with connective tissue disease-associated PAH. For the purpose of baseline haemodynamics, the results obtained at the most recent catheterization was used, in 12 cases this was between 6 and 24 months before study entry. However, where planned routine follow catheterization was performed within 8 weeks of study entry (n=11) this data was used as the baseline data.
Six-minute walk test
Six-minute walk tests were performed by all patients using a standardized protocol in accordance with the American Thoracic Society guidelines.29 Patients who were unable to perform the test were recorded as achieving a distance of 0 m.
WHO functional class assessment
Functional class assessment was recorded in accordance with the WHO functional classification from 1998.30
Blood sampling and assay
Blood samples were analysed for biochemistry (including renal function) and N-TproBNP levels. Samples were transported to the chemical pathology department in serum gel tubes at room temperature where they were centrifuged and either analysed immediately or stored at −20°C for later analysis. The serum N-TproBNP level was measured on the Roche Modular Analytics E-170 (Eleccys module) immunoassay analyser. The method of sandwich immunoassay using electrochemiluminescence detection, is the standard method used in our department and shows high reproducibility.
In the patients without PAH, the baseline N-TproBNP levels were taken at their first visit. In the patients with SSc-PAH, N-TproBNP levels were measured at entry and every 3 months thereafter during the year of follow-up.
Echocardiography
M-mode and 2D-echocardiography were performed using an Acuson (Sequoia C256/512) echocardiogram. Continuous wave Doppler signals were recorded with a ‘Doptek’ (Southampton, UK) 2.0 MHz transducer. The peak instantaneous pressure drop from the right ventricle to the right atrium was calculated for the peak signal velocity from the tricuspid regurgitant (TR) signal by the simplified Bernoulli equation. No estimate of right atrial pressure was added to the Bernoulli equation value obtained from the TR jet, as previously described.4
Statistical methods
As the primary aim was to assess the reproducibility of the 395 pg/mL cut-off identified previously, we initially assessed specificity, sensitivity, and positive and negative predictive values associated with this cut-off in our sample. Wilson method was used to find 95% confidence intervals (CIs). Then, sensitivity and specificity of different cut-off levels on N-TproBNP for diagnosis and exclusion of SSc-PAH were calculated. ROC curves were drawn to identify N-TproBNP levels which gave optimal sensitivities and specificities for diagnosis and exclusion of SSc-PAH.
The association of BNP with a number of known markers for the severity of SSc-PAH was assessed. For this analysis, only patients with a diagnosis of SSc-PAH were included. The initial outcome markers examined were tricuspid gradient based on echo, mean pulmonary artery pressure (mPAP), pulmonary vascular resistance (PVR) and cardiac index from right heart catheterization, SMWD, and WHO class. These markers were examined and found to be normally distributed. However, the N-TproBNP was log-transformed to the base 10 in order to achieve normality. Pearson's correlation coefficient was used to analyse the correlation with N-TproBNP levels. The association between N-TproBNP and survival was undertaken using Cox proportional hazards models. The proportional hazards assumption was assessed visually using stratified Kaplan–Meier plots and found to be adequate. Data are presented as mean (SD). All factors associated with survival with a P-value of less than 0.1 in the univariate analysis were included in an initial multivariable model. The final model was chosen using backward selection with exclusion criteria of P>0.05.
All statistical tests were two-sided and a P-value of less than 0.05 was nominally considered as statistically significant. We have made no formal adjustments for errors caused by multiple testing (Type 1 error), as there was a single primary aim for our study, which was to investigate the reproducibility of our previously found cut-off of 395 pg/mL. However, it is important to regard other analyses carried out in the light of the risk of type 1 error.
Follow-up
Survival data were available for all patients until the date of death (16 patients) or 13th May 2005. Standard survival methods were used to compare survival times between those with SSc-PAH and those without.
N-TproBNP was included as a time-updated variable. Thus, our model estimated the effect of the most recent N-TproBNP on the hazard of survival. For the purpose of analysis, an individual was assumed to have a constant N-TproBNP after the date it was measured until the date of the next measurement, at which time this value was taken.
Subsequent survival analyses focused on the predictive value of N-TproBNP levels in patients with SSc-PAH, and so analyses only included those with SSc-PAH. Cox proportional hazards models were used to analyse the association between N-TproBNP and survival. As patients had more than one N-TproBNP measurement, both N-TproBNP levels at the time of entry into the study and time-updated levels were included in the analyses.
For the purpose of analysis, an individual was assumed to have a constant N-TproBNP after the date it was measured until the date of the next measurement, at which time the value was updated.31
Results
A total of 109 patients were enrolled in this study. Sixty-eight patients had SSc-PAH confirmed at cardiac catheterization [mean age 60 (10) years]. Forty-one patients had SSc but normal tricuspid gradient on echocardiography (<25 mmHg). The mean estimated TG in this group was 20 (7) mmHg. Of the 41 patients, 11 of them had unexplained breathlessness and were enrolled after cardiac catheterization excluded PAH, LV systolic and diastolic dysfunction and coronary heart disease. The clinical profile and haemodynamic data are shown in Table 1.
Patient clinical profile and haemodynamics
| . | SSc-PAH (cases: n=68) . | SSc (no PAH) (controls: n=41) . |
|---|---|---|
| Mean age (years) | 60 (11) | 53 (12) |
| Gender M/F | 14/54 | 7/34 |
| mRAP (mmHg) | 8 (5) | 5 (2) |
| mPAP (mmHg) | 40 (12) | 21 (2) |
| MAP (mmHg) | 97 (18) | 96 (13) |
| PVR (dyn/s/cm−5) | 607 (402) | 218 (84) |
| Cardiac index (L/min) | 2.5 (0.6) | 2.8 (0.5) |
| Echo PAP (mmHg) | 54 (23) | 20 (7) |
| SMWD (m) | 242 (136) | 391 (107) |
| SMWD <150 (%) | 22 (33) | 1 (2) |
| SMWD >150<350 (%) | 29 (42) | 9 (23) |
| SMWD >350 (%) | 17 (25) | 29 (70.7) |
| WHO functional class | ||
| I (%) | 2 (2) | 19 (46) |
| II (%) | 13 (19) | 14 (34) |
| III (%) | 36 (53) | 7 (17) |
| IV (%) | 17 (25) | 1 (2) |
| Type of scleroderma | ||
| Limited (%) | 56 (82) | 34 (83) |
| Diffuse (%) | 12 (18) | 7 (17) |
| Patients with pulmonary fibrosis (%) | 21 (31) | 8 (20) |
| Serum creatinine (µmol/L) | 88 (19) | 76 (14) |
| N-TproBNP (log10 pg/mL) | 2.73 (0.65) | 1.93 (0.45) |
| N-TproBNP (pg/mL) | 1474 (2642) | 139 (150) |
| Individuals on PAH treatment (%) | 51 (75) | 41 (100) |
| Type of PAH treatment | ||
| Calcium-channel blockers (%) | 5 (7) | N/A |
| Bosentan (%) | 31 (46) | |
| Oral iloprost (%) | 2 (3) | |
| Inhaled iloprost (%) | 3 (4) | |
| Intravenous prostanoid (%) | 6 (9) | |
| Combination (bosentan + CCB or iloprost) (%) | 4 (6) | |
| . | SSc-PAH (cases: n=68) . | SSc (no PAH) (controls: n=41) . |
|---|---|---|
| Mean age (years) | 60 (11) | 53 (12) |
| Gender M/F | 14/54 | 7/34 |
| mRAP (mmHg) | 8 (5) | 5 (2) |
| mPAP (mmHg) | 40 (12) | 21 (2) |
| MAP (mmHg) | 97 (18) | 96 (13) |
| PVR (dyn/s/cm−5) | 607 (402) | 218 (84) |
| Cardiac index (L/min) | 2.5 (0.6) | 2.8 (0.5) |
| Echo PAP (mmHg) | 54 (23) | 20 (7) |
| SMWD (m) | 242 (136) | 391 (107) |
| SMWD <150 (%) | 22 (33) | 1 (2) |
| SMWD >150<350 (%) | 29 (42) | 9 (23) |
| SMWD >350 (%) | 17 (25) | 29 (70.7) |
| WHO functional class | ||
| I (%) | 2 (2) | 19 (46) |
| II (%) | 13 (19) | 14 (34) |
| III (%) | 36 (53) | 7 (17) |
| IV (%) | 17 (25) | 1 (2) |
| Type of scleroderma | ||
| Limited (%) | 56 (82) | 34 (83) |
| Diffuse (%) | 12 (18) | 7 (17) |
| Patients with pulmonary fibrosis (%) | 21 (31) | 8 (20) |
| Serum creatinine (µmol/L) | 88 (19) | 76 (14) |
| N-TproBNP (log10 pg/mL) | 2.73 (0.65) | 1.93 (0.45) |
| N-TproBNP (pg/mL) | 1474 (2642) | 139 (150) |
| Individuals on PAH treatment (%) | 51 (75) | 41 (100) |
| Type of PAH treatment | ||
| Calcium-channel blockers (%) | 5 (7) | N/A |
| Bosentan (%) | 31 (46) | |
| Oral iloprost (%) | 2 (3) | |
| Inhaled iloprost (%) | 3 (4) | |
| Intravenous prostanoid (%) | 6 (9) | |
| Combination (bosentan + CCB or iloprost) (%) | 4 (6) | |
Patient clinical profile and haemodynamics
| . | SSc-PAH (cases: n=68) . | SSc (no PAH) (controls: n=41) . |
|---|---|---|
| Mean age (years) | 60 (11) | 53 (12) |
| Gender M/F | 14/54 | 7/34 |
| mRAP (mmHg) | 8 (5) | 5 (2) |
| mPAP (mmHg) | 40 (12) | 21 (2) |
| MAP (mmHg) | 97 (18) | 96 (13) |
| PVR (dyn/s/cm−5) | 607 (402) | 218 (84) |
| Cardiac index (L/min) | 2.5 (0.6) | 2.8 (0.5) |
| Echo PAP (mmHg) | 54 (23) | 20 (7) |
| SMWD (m) | 242 (136) | 391 (107) |
| SMWD <150 (%) | 22 (33) | 1 (2) |
| SMWD >150<350 (%) | 29 (42) | 9 (23) |
| SMWD >350 (%) | 17 (25) | 29 (70.7) |
| WHO functional class | ||
| I (%) | 2 (2) | 19 (46) |
| II (%) | 13 (19) | 14 (34) |
| III (%) | 36 (53) | 7 (17) |
| IV (%) | 17 (25) | 1 (2) |
| Type of scleroderma | ||
| Limited (%) | 56 (82) | 34 (83) |
| Diffuse (%) | 12 (18) | 7 (17) |
| Patients with pulmonary fibrosis (%) | 21 (31) | 8 (20) |
| Serum creatinine (µmol/L) | 88 (19) | 76 (14) |
| N-TproBNP (log10 pg/mL) | 2.73 (0.65) | 1.93 (0.45) |
| N-TproBNP (pg/mL) | 1474 (2642) | 139 (150) |
| Individuals on PAH treatment (%) | 51 (75) | 41 (100) |
| Type of PAH treatment | ||
| Calcium-channel blockers (%) | 5 (7) | N/A |
| Bosentan (%) | 31 (46) | |
| Oral iloprost (%) | 2 (3) | |
| Inhaled iloprost (%) | 3 (4) | |
| Intravenous prostanoid (%) | 6 (9) | |
| Combination (bosentan + CCB or iloprost) (%) | 4 (6) | |
| . | SSc-PAH (cases: n=68) . | SSc (no PAH) (controls: n=41) . |
|---|---|---|
| Mean age (years) | 60 (11) | 53 (12) |
| Gender M/F | 14/54 | 7/34 |
| mRAP (mmHg) | 8 (5) | 5 (2) |
| mPAP (mmHg) | 40 (12) | 21 (2) |
| MAP (mmHg) | 97 (18) | 96 (13) |
| PVR (dyn/s/cm−5) | 607 (402) | 218 (84) |
| Cardiac index (L/min) | 2.5 (0.6) | 2.8 (0.5) |
| Echo PAP (mmHg) | 54 (23) | 20 (7) |
| SMWD (m) | 242 (136) | 391 (107) |
| SMWD <150 (%) | 22 (33) | 1 (2) |
| SMWD >150<350 (%) | 29 (42) | 9 (23) |
| SMWD >350 (%) | 17 (25) | 29 (70.7) |
| WHO functional class | ||
| I (%) | 2 (2) | 19 (46) |
| II (%) | 13 (19) | 14 (34) |
| III (%) | 36 (53) | 7 (17) |
| IV (%) | 17 (25) | 1 (2) |
| Type of scleroderma | ||
| Limited (%) | 56 (82) | 34 (83) |
| Diffuse (%) | 12 (18) | 7 (17) |
| Patients with pulmonary fibrosis (%) | 21 (31) | 8 (20) |
| Serum creatinine (µmol/L) | 88 (19) | 76 (14) |
| N-TproBNP (log10 pg/mL) | 2.73 (0.65) | 1.93 (0.45) |
| N-TproBNP (pg/mL) | 1474 (2642) | 139 (150) |
| Individuals on PAH treatment (%) | 51 (75) | 41 (100) |
| Type of PAH treatment | ||
| Calcium-channel blockers (%) | 5 (7) | N/A |
| Bosentan (%) | 31 (46) | |
| Oral iloprost (%) | 2 (3) | |
| Inhaled iloprost (%) | 3 (4) | |
| Intravenous prostanoid (%) | 6 (9) | |
| Combination (bosentan + CCB or iloprost) (%) | 4 (6) | |
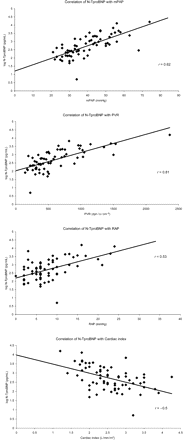
Correlation of plasma N-TproBNP levels with cardiopulmonary haemodynamics
N-TproBNP levels in patients with SSc-PAH were significantly higher than in those without PAH [1474 (2642) pg/mL vs. 139 (151) pg/mL, P=0.0002]. In patients with SSc-PAH, N-TproBNP positively and significantly correlated with mPAP (r=0.62; P<0.0001), PVR (r=0.81; P<0.0001), and RAP (r=0.53; P<0.0001). N-TproBNP inversely and significantly correlated with cardiac index (r=−0.5; P<0.0001). (Figure 1).
Graphs showing correlation of plasma N-TproBNP levels with cardiopulmonary haemodynamics (mPAP, PVR, RAP, and cardiac index).
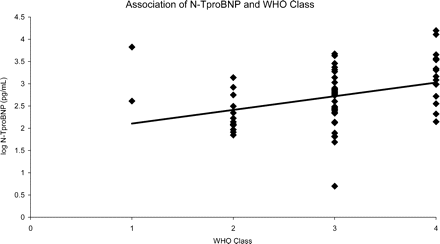
Association of plasma N-TproBNP levels with WHO class
In patients with SSc-PAH, the mean (SD) N-TproBNP level in the 53 (78%) patients with WHO classes III and IV dyspnoea was significantly higher than in the 13 (19%) patients with WHO class II dyspnoea [1677 (2835) pg/mL vs. 325 (388) pg/mL; P=0.02] (Figure 2).
Association of N-TproBNP with WHO class in patients with SSc-PAH.
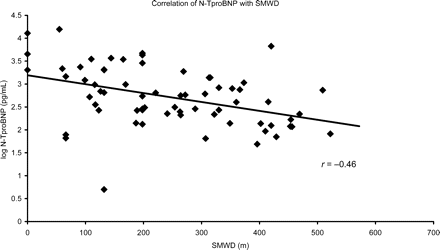
Correlation of plasma N-TproBNP levels with exercise capacity
There was a significant inverse correlation of N-TproBNP levels with SMWD. The mean (SD) N-TproBNP in patients with an SMWD less than 350 m was 1478 (2691) pg/mL compared with 326 (989) pg/mL in patients with an SMWD greater than 350 m (P<0.0001). For every 100 m further an individual could walk, there was a significant fall in N-TproBNP levels (Figure 3).
The diagnostic ability of BNP to predict the presence of SSc-PAH
Only two of the 41 patients without SSc-PAH had a level of N-TproBNP >395 pg/mL, giving a specificity of 95.1% (95% CI 83.9, 98.7%). Of the 68 patients, 38 with SSc-PAH had a level greater than this giving a sensitivity of 55.9% (95% CI 44.1, 67.4%). Of the 40, 38 patients who had a level greater than 395 pg/mL had SSc-PAH giving a positive predictive value of 95.1% (95% CI 83.9, 98.7%). (Table 2).
Sensitivity, specificity, positive, and negative predictive value of N-TproBNP for predicting SSc-PAH
| . | Level of BNP . | ||
|---|---|---|---|
| . | >395 units . | ≤395 units . | Total . |
| PAH present (cases) | 38 | 30 | 68 |
| PAH absent (controls) | 2 | 39 | 41 |
| Total | 40 | 69 | 109 |
| . | Level of BNP . | ||
|---|---|---|---|
| . | >395 units . | ≤395 units . | Total . |
| PAH present (cases) | 38 | 30 | 68 |
| PAH absent (controls) | 2 | 39 | 41 |
| Total | 40 | 69 | 109 |
Sensitivity: 38/68=55.9% (95% CI 44.1, 67.4%).
Positive predictive value: 38/40=95.1% (95% CI 83.9, 98.7%).
Specificity: 39/41=95.1% (95% CI 83.9, 98.7%).
Negative predictive value: 39/69=56.5% (95% CI 44.8, 67.6%).
Sensitivity, specificity, positive, and negative predictive value of N-TproBNP for predicting SSc-PAH
| . | Level of BNP . | ||
|---|---|---|---|
| . | >395 units . | ≤395 units . | Total . |
| PAH present (cases) | 38 | 30 | 68 |
| PAH absent (controls) | 2 | 39 | 41 |
| Total | 40 | 69 | 109 |
| . | Level of BNP . | ||
|---|---|---|---|
| . | >395 units . | ≤395 units . | Total . |
| PAH present (cases) | 38 | 30 | 68 |
| PAH absent (controls) | 2 | 39 | 41 |
| Total | 40 | 69 | 109 |
Sensitivity: 38/68=55.9% (95% CI 44.1, 67.4%).
Positive predictive value: 38/40=95.1% (95% CI 83.9, 98.7%).
Specificity: 39/41=95.1% (95% CI 83.9, 98.7%).
Negative predictive value: 39/69=56.5% (95% CI 44.8, 67.6%).
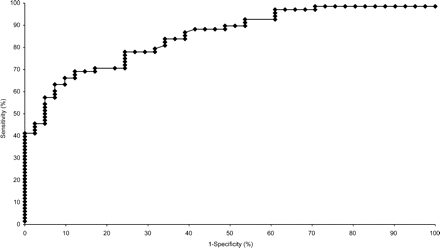
Figure 4 shows an ROC curve of N-TproBNP to predict the presence of SSc-PAH. A sensitivity of 90% (95% CI 80, 95%) was achieved at a cut-off N-TproBNP level of 91 pg/mL. This level gives a specificity of 51% (95% CI 37, 66%) and positive and negative predictive values of 75% (95% CIs 65, 83, 57, and 87%, respectively).
ROC curves of the ability of N-TproBNP to predict the presence of SSc-PAH. A sensitivity of 90%, corresponds with a cut-off N-TproBNP of 91 pg/mL. This gives a specificity of 51% and positive and negative predictive values of 75%, respectively.
Survival with SSc-PAH
All patients with SSc-PAH confirmed at right heart catheterization were followed for 1 year. There were 16 deaths during the follow-up period. The survival rates at 6 months and 1 year were 90% (95% CI 82, 97%) and 84% (95% CI 75, 92%; Kaplan–Meier estimates).
Changes in N-TproBNP and survival
N-TproBNP levels were measured in 52 patients with SSc-PAH at three-monthly intervals during the 1 year follow-up period. Baseline N-TproBNP, change in N-TproBNP, baseline cardiopulmonary haemodynamics, scleroderma-subset (whether limited or diffuse type), age, gender, ethnicity, WHO class, and SMWD were included in a Cox proportional hazards model (Table 3). Baseline N-TproBNP and change in N-TproBNP significantly predicted survival in both univariable and multivariable analyses. For every 10-fold increase in baseline N-TproBNP level there was a five-fold increased risk of dying [Hazard ratio (HR)=4.82; 95% CI 1.29, 18.05; P=0.002]. For every 10-fold increase in follow-up N-TproBNP (from baseline) there was a four-fold increased risk of dying (HR=3.82; 95% CI 1.46, 9.96; P=0.006). Gender was also significantly associated with survival, male patients being five times more likely to die than female patients (HR=5.07; 95% CI 1.81, 14.16; P=0.002). Ethnicity, scleroderma subtype, age, and baseline haemodynamic parameters were not found to be significantly associated with survival in this study in either univariable or multivariable analysis.
Cox proportional hazards model of factors associated with survival in SSc-PAH. Univariable and multivariable analyses
| Baseline BNP . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|
| . | Relative hazard . | 95% CI . | P-value . | Relative hazard . | 95% CI . | P-value . |
| Change in BNP from baseline to most recent value | ||||||
| Per 1 log10 units higher | 2.19 | 1.03, 4.69 | 0.04 | 4.82 | 1.29, 18.05 | 0.002 |
| Per 1 log10 units higher | 3.16 | 0.94, 10.61 | 0.063 | 3.82 | 1.46, 9.96 | 0.0062 |
| Ethnicity | ||||||
| Caucasian | 0.59 | 0.13, 2.58 | 0.48 | |||
| Other | 1.00 (ref) | — | ||||
| Scleroderma | ||||||
| Limited | 0.62 | 0.20, 1.94 | 0.41 | |||
| Diffused | 1.00 (ref) | |||||
| Baseline age | ||||||
| Per 10 years older | 1.22 | 0.82, 1.82 | 0.32 | |||
| Gender | ||||||
| Male | 4.54 | 1.70, 12.12 | 0.0026 | 5.07 | 1.81, 14.16 | 0.0020 |
| Female | 1.00 | — | 1.00 | — | ||
| Baseline PVR | ||||||
| Per 100 higher | 1.05 | 0.96, 1.16 | 0.28 | |||
| Baseline cardiac index | ||||||
| Per 0.2 higher | 0.93 | 0.79, 1.09 | 0.36 | |||
| Baseline mPAP | ||||||
| Per 5 higher | 1.04 | 0.86, 1.27 | 0.68 | |||
| Baseline RAP | ||||||
| Per 1 higher | 1.01 | 0.92, 1.10 | 0.91 | |||
| Baseline SAO2 | ||||||
| Per 5 higher | 0.94 | 0.74, 1.20 | 0.63 | |||
| Baseline WHO group | ||||||
| 1 and 2 | 1.00 | — | 0.055 | |||
| 3 and 4 | 2.13 | 0.98, 4.61 | ||||
| Baseline 6 min walk time | ||||||
| Per 100 m further | 0.63 | 0.41, 0.96 | 0.032 | |||
| Baseline BNP . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|
| . | Relative hazard . | 95% CI . | P-value . | Relative hazard . | 95% CI . | P-value . |
| Change in BNP from baseline to most recent value | ||||||
| Per 1 log10 units higher | 2.19 | 1.03, 4.69 | 0.04 | 4.82 | 1.29, 18.05 | 0.002 |
| Per 1 log10 units higher | 3.16 | 0.94, 10.61 | 0.063 | 3.82 | 1.46, 9.96 | 0.0062 |
| Ethnicity | ||||||
| Caucasian | 0.59 | 0.13, 2.58 | 0.48 | |||
| Other | 1.00 (ref) | — | ||||
| Scleroderma | ||||||
| Limited | 0.62 | 0.20, 1.94 | 0.41 | |||
| Diffused | 1.00 (ref) | |||||
| Baseline age | ||||||
| Per 10 years older | 1.22 | 0.82, 1.82 | 0.32 | |||
| Gender | ||||||
| Male | 4.54 | 1.70, 12.12 | 0.0026 | 5.07 | 1.81, 14.16 | 0.0020 |
| Female | 1.00 | — | 1.00 | — | ||
| Baseline PVR | ||||||
| Per 100 higher | 1.05 | 0.96, 1.16 | 0.28 | |||
| Baseline cardiac index | ||||||
| Per 0.2 higher | 0.93 | 0.79, 1.09 | 0.36 | |||
| Baseline mPAP | ||||||
| Per 5 higher | 1.04 | 0.86, 1.27 | 0.68 | |||
| Baseline RAP | ||||||
| Per 1 higher | 1.01 | 0.92, 1.10 | 0.91 | |||
| Baseline SAO2 | ||||||
| Per 5 higher | 0.94 | 0.74, 1.20 | 0.63 | |||
| Baseline WHO group | ||||||
| 1 and 2 | 1.00 | — | 0.055 | |||
| 3 and 4 | 2.13 | 0.98, 4.61 | ||||
| Baseline 6 min walk time | ||||||
| Per 100 m further | 0.63 | 0.41, 0.96 | 0.032 | |||
Cox proportional hazards model of factors associated with survival in SSc-PAH. Univariable and multivariable analyses
| Baseline BNP . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|
| . | Relative hazard . | 95% CI . | P-value . | Relative hazard . | 95% CI . | P-value . |
| Change in BNP from baseline to most recent value | ||||||
| Per 1 log10 units higher | 2.19 | 1.03, 4.69 | 0.04 | 4.82 | 1.29, 18.05 | 0.002 |
| Per 1 log10 units higher | 3.16 | 0.94, 10.61 | 0.063 | 3.82 | 1.46, 9.96 | 0.0062 |
| Ethnicity | ||||||
| Caucasian | 0.59 | 0.13, 2.58 | 0.48 | |||
| Other | 1.00 (ref) | — | ||||
| Scleroderma | ||||||
| Limited | 0.62 | 0.20, 1.94 | 0.41 | |||
| Diffused | 1.00 (ref) | |||||
| Baseline age | ||||||
| Per 10 years older | 1.22 | 0.82, 1.82 | 0.32 | |||
| Gender | ||||||
| Male | 4.54 | 1.70, 12.12 | 0.0026 | 5.07 | 1.81, 14.16 | 0.0020 |
| Female | 1.00 | — | 1.00 | — | ||
| Baseline PVR | ||||||
| Per 100 higher | 1.05 | 0.96, 1.16 | 0.28 | |||
| Baseline cardiac index | ||||||
| Per 0.2 higher | 0.93 | 0.79, 1.09 | 0.36 | |||
| Baseline mPAP | ||||||
| Per 5 higher | 1.04 | 0.86, 1.27 | 0.68 | |||
| Baseline RAP | ||||||
| Per 1 higher | 1.01 | 0.92, 1.10 | 0.91 | |||
| Baseline SAO2 | ||||||
| Per 5 higher | 0.94 | 0.74, 1.20 | 0.63 | |||
| Baseline WHO group | ||||||
| 1 and 2 | 1.00 | — | 0.055 | |||
| 3 and 4 | 2.13 | 0.98, 4.61 | ||||
| Baseline 6 min walk time | ||||||
| Per 100 m further | 0.63 | 0.41, 0.96 | 0.032 | |||
| Baseline BNP . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|
| . | Relative hazard . | 95% CI . | P-value . | Relative hazard . | 95% CI . | P-value . |
| Change in BNP from baseline to most recent value | ||||||
| Per 1 log10 units higher | 2.19 | 1.03, 4.69 | 0.04 | 4.82 | 1.29, 18.05 | 0.002 |
| Per 1 log10 units higher | 3.16 | 0.94, 10.61 | 0.063 | 3.82 | 1.46, 9.96 | 0.0062 |
| Ethnicity | ||||||
| Caucasian | 0.59 | 0.13, 2.58 | 0.48 | |||
| Other | 1.00 (ref) | — | ||||
| Scleroderma | ||||||
| Limited | 0.62 | 0.20, 1.94 | 0.41 | |||
| Diffused | 1.00 (ref) | |||||
| Baseline age | ||||||
| Per 10 years older | 1.22 | 0.82, 1.82 | 0.32 | |||
| Gender | ||||||
| Male | 4.54 | 1.70, 12.12 | 0.0026 | 5.07 | 1.81, 14.16 | 0.0020 |
| Female | 1.00 | — | 1.00 | — | ||
| Baseline PVR | ||||||
| Per 100 higher | 1.05 | 0.96, 1.16 | 0.28 | |||
| Baseline cardiac index | ||||||
| Per 0.2 higher | 0.93 | 0.79, 1.09 | 0.36 | |||
| Baseline mPAP | ||||||
| Per 5 higher | 1.04 | 0.86, 1.27 | 0.68 | |||
| Baseline RAP | ||||||
| Per 1 higher | 1.01 | 0.92, 1.10 | 0.91 | |||
| Baseline SAO2 | ||||||
| Per 5 higher | 0.94 | 0.74, 1.20 | 0.63 | |||
| Baseline WHO group | ||||||
| 1 and 2 | 1.00 | — | 0.055 | |||
| 3 and 4 | 2.13 | 0.98, 4.61 | ||||
| Baseline 6 min walk time | ||||||
| Per 100 m further | 0.63 | 0.41, 0.96 | 0.032 | |||
Discussion
Value of N-TproBNP in screening for SSc-PAH
This study confirms our previous finding that a cut-off value at or >395 pg/mL, N-TproBNP strongly supports the diagnosis of SSc-PAH in patients where this diagnosis is suspected, with a positive predictive value of 95% and modest negative predictive value. We have also shown that, in a small control population, only at very low levels of N-TproBNP can one consider this a ‘rule out’ test. Although further work is necessary to evaluate the role of N-TproBNP as a screening investigation, it is clear that the positive and negative predictive values are similar to those observed with echocardiography.4
Prognostic value of N-TproBNP
In this study, N-TproBNP predicted survival in patients with SSc-PAH. Both the baseline level and change in N-TproBNP predicted survival; the higher the level, the worse the prognosis. The 6 months and 1 year survival of patients with a level below the median (553 pg/mL) were 97 and 96% respectively, and 82 and 73% for patients with a level greater than this.
Relationship of N-TproBNP to predictors of survival in PAH
Significant correlations were observed between N-TproBNP levels and cardiopulmonary haemodynamics, WHO functional class, and SMWD. We have previously shown that survival in patients with SSc-PAH is related to cardiopulmonary haemodynamics.5 These data may explain why raised levels of N-TproBNP were associated with an adverse prognosis in this study. We have shown that N-TproBNP levels independently and incrementally predict survival in SSc-PAH.
Use of N-TproBNP to monitor disease
Disease progression in SSc-PAH and trials of drug efficacy in PAH are monitored using WHO functional class and the SMWD test. Both these assessments have their limitations as they are not objective. In patients with SSc-PAH, perception of functional status and performance during the SMWD may be affected by their general musculoskeletal, myocardial, and pulmonary interstitial involvement. The fact that N-TproBNP correlates with WHO functional class and SMWD in a population with SSc-PAH, suggests that the dominant influence on these factors in this selected population is indeed right heart strain. N-TproBNP is less influenced by other factors than functional status and SMWT and thus, provides an attractive alternative for monitoring disease progression and response to therapy.
N-TproBNP in right heart failure
In heart failure and myocardial infarction, raised plasma BNP levels are a well-validated and established marker of disease state and indicate a poor prognosis.32 BNP levels are now recommended as part of the routine evaluation of patients with left heart failure.33 There is limited information on the use of BNP in right heart failure, particularly in a sizeable and homogenous group of patients.21–27 In a study of 28 patients with iPAH, single BNP levels correlated with right heart haemodynamics, WHO functional class, and SMWD but there was no follow-up data. Therefore, although the authors concluded that BNP was an excellent marker for assessing functional impairment in IPAH because of right heart failure, they were unable to show that BNP may also be of value as a marker of disease progression.21 Only Nayaga et al. has shown an association with survival in IPAH relying on a binary approach of considering whether levels were above or below the median. BNP levels change rapidly in response to exercise and thus, in patients with significant dyspnoea BNP, as a test, has significant practical limitations. Our data enhances previous data, by showing that the more stable analogue (N-TproBNP) can be used and by showing that the relationship with prognosis is log-linear both at baseline in response to change in N-TproBNP level, and is thus applicable in all patients, irrespective of the median level in the population.
Proposed mechanisms of N-TproBNP release
Factors leading to the release of BNP include endothelin-1,34 angiotensin-II,35 and glucocorticoids.36 It is synthesised as an inactive precursor preproBNP, which is subsequently cleaved to form proBNP. This is further split to form the active BNP and inactive N-terminal fragment (N-TproBNP).37 BNP acts via natriuretic peptide receptors (NPR). These are transmembrane receptors which use cyclic 3′, 5′-guanosine monophosphate (cGMP) as the intracellular second messenger.38 BNP binds to both NPR-A and NPR-C.39 NPR-C is involved in the breakdown and clearance of natriuretic peptides from the circulation.40 Through its interaction with NPR-A, BNP promotes intravascular volume contraction41 and hypotension,42 promotes diuresis43 (antagonizing the renin–angiotensin–aldosterone system).44
Limitations
We have assessed the optimal cut-off for sensitivity and specificity of N-TproBNP in a single study, and thus our cut-off may be sensitive to the specific study population. Indeed one of our primary aims was to assess whether the 395 pg/mL cut-off identified to investigate this further, such as boot strapping, are beyond the scope of this study.
This study was not designed to, or sufficiently powered to investigate the role of N-TproBNP as a ‘rule-out test’. More control patients would need to be included for this analysis. The majority of patients with PAH were already on advanced therapies and so this may confound the interpretation of N-TproBNP levels as a diagnostic test, as we do not know whether these therapies have a direct effect on BNP levels. However, this would not appreciably affect interpretation of N-TproBNP levels as a prognostic test. We did not look at the effect of treatment or no treatment on N-TproBNP levels. N-TproBNP levels are however, known to fall in response to effective disease-modifying therapy (Bosentan) in patients with IPAH,45 correlating this fall with improved survival would have immense implications for future treatment monitoring.
Conclusions
In patients identified as having probable SScPAH on echocardiography, an N-TproBNP level >395pg/mL strongly supports the diagnosis. Baseline and change in N-TproBNP levels identify patients with SSc-PAH who have a particularly adverse prognosis who may benefit from the introduction of or modification of advanced therapies. For every 10-fold change in N-TproBNP level up or down then prognosis worsens or improves nearly four-fold. N-TproBNP is the most powerful non-invasive prognostic tool identified to date for patients with SSc-PAH. Further work is required to demonstrate whether falling levels observed in response to therapy, consistently translate into early confirmation of beneficial disease modification. However, increasing levels despite treatment, is associated with an adverse prognosis and should lead to modification of the therapeutic strategy.
N-TproBNP identifies patients with SSc-PAH with a similar positive and negative predictive values to that seen with echocardiography. Establishing N-TproBNP as a screening tool for this population will require further work, in particular, a large-scale study in a population with SSc but without known PAH.
Acknowledgements
I would like the thank Miss Elizabeth Neville for her help with the biochemical analyses.
Conflict of interest: D.N. is in receipt of a grant from Roche Pharmaceuticals. There are no other potential conflicts of interest.