Key Points
-
Multiple independent associations within the HLA 8.1 ancestral haplotype are the strongest genetic risk factors for idiopathic inflammatory myopathies (IIMs).
-
Although some associations overlap with those for other autoimmune diseases, other genetic risk factors are unique to certain IIM phenotypes, suggesting that these phenotypes have different pathophysiologies.
-
In addition to drug-induced myositis, epidemiological data support a role for infections, prior lung disease, physical exertion, collagen implants, ultraviolet radiation and smoking in the development of IIM phenotypes.
-
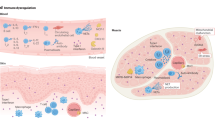
Although the disease mechanisms for IIM are ill-defined, both the innate immune system (including cytokines and chemokines) and adaptive immune system (including autoantibodies and antigen-specific T cells) are probably involved.
-
Several non-immune-mediated mechanisms contribute to IIM pathogenesis, including cell-stress pathways, free radicals, altered energy metabolism, protein homeostasis and mitochondrial damage.
-
Multidisciplinary collaborative approaches, focused use of resources and better investigative tools are needed to define additional risk and protective factors and pathogenic mechanisms, to cure and prevent the development of IIM.
Abstract
Autoimmune diseases develop as a result of chronic inflammation owing to interactions between genes and the environment. However, the mechanisms by which autoimmune diseases evolve remain poorly understood. Newly discovered risk factors and pathogenic processes in the various idiopathic inflammatory myopathy (IIM) phenotypes (known collectively as myositis) have illuminated innovative approaches for understanding these diseases. The HLA 8.1 ancestral haplotype is a key risk factor for major IIM phenotypes in some populations, and several genetic variants associated with other autoimmune diseases have been identified as IIM risk factors. Environmental risk factors are less well studied than genetic factors but might include viruses, bacteria, ultraviolet radiation, smoking, occupational and perinatal exposures and a growing list of drugs (including biologic agents) and dietary supplements. Disease mechanisms vary by phenotype, with evidence of shared innate and adaptive immune and metabolic pathways in some phenotypes but unique pathways in others. The heterogeneity and rarity of the IIMs make advancements in diagnosis and treatment cumbersome. Novel approaches, better-defined phenotypes, and international, multidisciplinary consensus have contributed to progress, and it is hoped that these methods will eventually enable therapeutic intervention before the onset or major progression of disease. In the future, preemptive strategies for IIM management might be possible.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lundberg, I. E., de Visser, M. & Werth, V. P. Classification of myositis. Nat. Rev. Rheumatol. https://doi.org/nrrheum.2018.41 (2018).
Rider, L. G. et al. Update on outcome assessment in myositis. Nat. Rev. Rheumatol. https://doi.org/nrrheum.2018.33 (2018).
McHugh, N.J. & Tansley, S.L. Autoantibodies in myositis Nat. Rev. Rheumatol. https://doi.org/10.1038/nrrheum.2018.56 (2018).
Oddis, C. V. & Aggarwal, R. Treatment in myositis. Nat. Rev. Rheumatol. https://doi.org/10.1038/nrrheum.2018.42 (2018).
Ozaki, T. et al. Two patients in the same family with anti-ARS antibody-associated myositis. Mod. Rheumatol. 24, 699–700 (2014).
Rider, L. G. et al. Clinical, serologic, and immunogenetic features of familial idiopathic inflammatory myopathy. Arthritis Rheum. 41, 710–719 (1998).
Hyttinen, V., Kaprio, J., Kinnunen, L., Koskenvuo, M. & Tuomilehto, J. Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a nationwide follow-up study. Diabetes 52, 1052–1055 (2003).
van der Woude, D. et al. Quantitative heritability of anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis. Arthritis Rheum. 60, 916–923 (2009).
Ginn, L. R. et al. Familial autoimmunity in pedigrees of idiopathic inflammatory myopathy patients suggests common genetic risk factors for many autoimmune diseases. Arthritis Rheum. 41, 400–405 (1998).
Niewold, T. B., Wu, S. C., Smith, M., Morgan, G. A. & Pachman, L. M. Familial aggregation of autoimmune disease in juvenile dermatomyositis. Pediatrics 127, e1239–e1246 (2011).
Kuo, C. F. et al. Familial aggregation of systemic lupus erythematosus and coaggregation of autoimmune diseases in affected families. JAMA Intern. Med. 175, 1518–1526 (2015).
Kuo, C. F. et al. Familial risk of systemic sclerosis and co-aggregation of autoimmune diseases in affected families. Arthritis Res. Ther. 18, 231 (2016).
Meyer, A. et al. Incidence and prevalence of inflammatory myopathies: a systematic review. Rheumatology 54, 50–63 (2015).
Miller, F. W. et al. Genome-wide association study of dermatomyositis reveals genetic overlap with other autoimmune disorders. Arthritis Rheum. 65, 3239–3247 (2013).
Miller, F. W. et al. Genome-wide association study identifies HLA 8.1 ancestral haplotype alleles as major genetic risk factors for myositis phenotypes. Genes Immun. 16, 470–480 (2015).
Jia, X. et al. Imputing amino acid polymorphisms in human leukocyte antigens. PLoS ONE 8, e64683 (2013).
Rothwell, S. et al. Dense genotyping of immune-related loci in idiopathic inflammatory myopathies confirms HLA alleles as the strongest genetic risk factor and suggests different genetic background for major clinical subgroups. Ann. Rheum. Dis. 75, 1558–1566 (2016).
Rothwell, S. et al. Immune-array analysis in sporadic inclusion body myositis reveals HLA-DRB1 amino acid heterogeneity across the myositis spectrum. Arthritis Rheumatol. 69, 1090–1099 (2017).
Furuya, T. et al. Immunogenetic features in 120 Japanese patients with idiopathic inflammatory myopathy. J. Rheumatol. 31, 1768–1774 (2004).
Gao, X. et al. HLA class II alleles may influence susceptibility to adult dermatomyositis and polymyositis in a Han Chinese population. BMC Dermatol. 14, 9 (2014).
O'Hanlon, T. P. et al. Immunogenetic risk and protective factors for the idiopathic inflammatory myopathies: distinct HLA-A, -B, -Cw, -DRB1, and -DQA1 allelic profiles distinguish European American patients with different myositis autoantibodies. Medicine 85, 111–127 (2006).
O'Hanlon, T. P. & Miller, F. W. Genetic risk and protective factors for the idiopathic inflammatory myopathies. Curr. Rheumatol. Rep. 11, 287–294 (2009).
Mammen, A. L. et al. Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Care Res. 64, 1233–1237 (2012).
Kishi, T. et al. Association of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase autoantibodies with DRB1*07:01 and severe myositis in juvenile myositis patients. Arthritis Care Res. 69, 1088–1094 (2017).
Chinoy, H. et al. The protein tyrosine phosphatase N22 gene is associated with juvenile and adult idiopathic inflammatory myopathy independent of the HLA 8.1 haplotype in British Caucasian patients. Arthritis Rheum. 58, 3247–3254 (2008).
Sugiura, T. et al. Positive association between STAT4 polymorphisms and polymyositis/dermatomyositis in a Japanese population. Ann. Rheum. Dis. 71, 1646–1650 (2012).
Wang, Q. et al. Positive association of genetic variations in the phospholipase C-like 1 gene with dermatomyositis in Chinese Han. Immunol. Res. 64, 204–212 (2016).
Lintner, K. E. et al. Gene copy-number variations (CNVs) of complement C4 and C4A deficiency in genetic risk and pathogenesis of juvenile dermatomyositis. Ann. Rheum. Dis. 75, 1599–1606 (2016).
Gang, Q. et al. Rare variants in SQSTM1 and VCP genes and risk of sporadic inclusion body myositis. Neurobiol. Aging 47, 218.e1–218.e9 (2016).
Weihl, C. C. et al. Targeted sequencing and identification of genetic variants in sporadic inclusion body myositis. Neuromuscul. Disord. 25, 289–296 (2015).
Guttsches, A. K. et al. Proteomics of rimmed vacuoles define new risk allele in inclusion body myositis. Ann. Neurol. 81, 227–239 (2017).
Rothwell, S., Lamb, J. A. & Chinoy, H. New developments in genetics of myositis. Curr. Opin. Rheumatol. 28, 651–656 (2016).
Torrelo, A. CANDLE syndrome as a paradigm of proteasome-related autoinflammation. Front. Immunol. 8, 927 (2017).
Wakeland, E. K., Liu, K., Graham, R. R. & Behrens, T. W. Delineating the genetic basis of systemic lupus erythematosus. Immunity 15, 397–408 (2001).
Svendsen, A. J. et al. On the origin of rheumatoid arthritis: the impact of environment and genes — a population based twin study. PLoS ONE 8, e57304 (2013).
Love, L. A. & Miller, F. W. Noninfectious environmental agents associated with myopathies. Curr. Opin. Rheumatol. 5, 712–718 (1993).
Reed, A. M. & Ytterberg, S. R. Genetic and environmental risk factors for idiopathic inflammatory myopathies. Rheum. Dis. Clin. North Am. 28, 891–916 (2002).
Miller, F. W. in The Autoimmune Diseases Vol. 4 (eds Rose, N. R. & Mackay, I. R.) 297–308 (Elsevier, 2006).
Miller, F. W. et al. Approaches for identifying and defining environmentally associated rheumatic disorders. Arthritis Rheum. 43, 243–249 (2000).
Miller, F. W. et al. Criteria for environmentally associated autoimmune diseases. J. Autoimmun. 39, 253–258 (2012).
Fazeli Farsani, S. et al. Increasing trends in the incidence and prevalence rates of type 1 diabetes among children and adolescents in the Netherlands. Pediatr. Diabetes 17, 44–52 (2016).
Bach, J. F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347, 911–920 (2002).
Leff, R. L. et al. Distinct seasonal patterns in the onset of adult idiopathic inflammatory myopathy in patients with anti-Jo-1 and anti-signal recognition particle autoantibodies. Arthritis Rheum. 34, 1391–1396 (1991).
Willer, C. J. et al. Timing of birth and risk of multiple sclerosis: population based study. BMJ 330, 120 (2005).
Sarkar, K. et al. Seasonal influence on the onset of idiopathic inflammatory myopathies in serologically defined groups. Arthritis Rheum. 52, 2433–2438 (2005).
Vegosen, L. J. et al. Seasonal birth patterns in myositis subgroups suggest an etiologic role of early environmental exposures. Arthritis Rheum. 56, 2719–2728 (2007).
Rose, N. R. The role of infection in the pathogenesis of autoimmune disease. Semin. Immunol. 10, 5–13 (1998).
Oldstone, M. B. Viruses and autoimmune diseases. Scand. J. Immunol. 46, 320–325 (1997).
Germolec, D., Kono, D. H., Pfau, J. C. & Pollard, K. M. Animal models used to examine the role of the environment in the development of autoimmune disease: findings from an NIEHS Expert Panel Workshop. J. Autoimmun. 39, 285–293 (2012).
Miller, F. W. Environmental agents and autoimmune diseases. Adv. Exp. Med. Biol. 711, 61–81 (2011).
Knight, J. C. Genomic modulators of the immune response. Trends Genet. 29, 74–83 (2013).
Brodin, P. et al. Variation in the human immune system is largely driven by non-heritable influences. Cell 160, 37–47 (2015).
Gourley, M. & Miller, F. W. Mechanisms of disease: environmental factors in the pathogenesis of rheumatic disease. Nat. Clin. Pract. Rheumatol. 3, 172–180 (2007).
Miller, F. W. et al. Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J. Autoimmun. 39, 259–271 (2012).
Mamyrova, G. et al. Environmental factors associated with disease flare in juvenile and adult dermatomyositis. Rheumatology 56, 1342–1347 (2017).
Holers, V. M. Insights from populations at risk for the future development of classified rheumatoid arthritis. Rheum. Dis. Clin. North Am. 40, 605–620 (2014).
Miller, F. W. in Inflammatory Myopathies (ed. Kagen, L.) 15–28 (Humana Press, 2009).
Gan, L. & Miller, F. W. State of the art: what we know about infectious agents and myositis. Curr. Opin. Rheumatol. 23, 585–594 (2011).
Allen, J. A. et al. Post-epidemic eosinophilia-myalgia syndrome associated with L-tryptophan. Arthritis Rheum. 63, 3633–3639 (2011).
Cukier, J. et al. Association between bovine collagen dermal implants and a dermatomyositis or a polymyositis-like syndrome. Ann. Intern. Med. 118, 920–928 (1993).
O'Hanlon, T. et al. Immunogenetic differences between Caucasian women with and those without silicone implants in whom myositis develops. Arthritis Rheum. 50, 3646–3650 (2004).
Shah, M., Targoff, I. N., Rice, M. M., Miller, F. W. & Rider, L. G. Brief report: ultraviolet radiation exposure is associated with clinical and autoantibody phenotypes in juvenile myositis. Arthritis Rheum. 65, 1934–1941 (2013).
Love, L. A. et al. Ultraviolet radiation intensity predicts the relative distribution of dermatomyositis and anti-Mi-2 autoantibodies in women. Arthritis Rheum. 60, 2499–2504 (2009).
Webber, M. P. et al. Nested case–control study of selected systemic autoimmune diseases in World Trade Center rescue/recovery workers. Arthritis Rheumatol. 67, 1369–1376 (2015).
Labirua-Iturburu, A. et al. Occupational exposure in patients with the antisynthetase syndrome. Clin. Rheumatol. 33, 221–225 (2014).
Nojima, T. et al. A case of polymyositis associated with hepatitis B infection. Clin. Exp. Rheumatol. 18, 86–88 (2000).
Chou, J. W., Lin, Y. L., Cheng, K. S., Wu, P. Y. & Reanne Ju, T. Dermatomyositis induced by hepatitis B virus-related hepatocellular carcinoma: a case report and review of the literature. Intern. Med. 56, 1831–1837 (2017).
Uruha, A. et al. Hepatitis C virus infection in inclusion body myositis: a case-control study. Neurology 86, 211–217 (2016).
Johnson, R. W., Williams, F. M., Kazi, S., Dimachkie, M. M. & Reveille, J. D. Human immunodeficiency virus-associated polymyositis: a longitudinal study of outcome. Arthritis Rheum. 49, 172–178 (2003).
Carroll, M. B. & Holmes, R. Dermatomyositis and HIV infection: case report and review of the literature. Rheumatol. Int. 31, 673–679 (2011).
Dalakas, M. C. et al. Inclusion body myositis with human immunodeficiency virus infection: four cases with clonal expansion of viral-specific T cells. Ann. Neurol. 61, 466–475 (2007).
Matsuura, E. et al. Inclusion body myositis associated with human T-lymphotropic virus-type I infection: eleven patients from an endemic area in Japan. J. Neuropathol. Exp. Neurol. 67, 41–49 (2008).
Calore, E. E. et al. Skeletal muscle pathology in 2 siblings infected with Toxoplasma gondii . J. Rheumatol. 27, 1556–1559 (2000).
Carroll, G. J., Will, R. K., Peter, J. B., Garlepp, M. J. & Dawkins, R. L. Penicillamine induced polymyositis and dermatomyositis. J. Rheumatol. 14, 995–1001 (1987).
Somani, A. K., Swick, A. R., Cooper, K. D. & McCormick, T. S. Severe dermatomyositis triggered by interferon beta-1a therapy and associated with enhanced type I interferon signaling. Arch. Dermatol. 144, 1341–1349 (2008).
Liu, S. W. et al. Dermatomyositis induced by anti-tumor necrosis factor in a patient with juvenile idiopathic arthritis. JAMA Dermatol. 149, 1204–1208 (2013).
Jones, J. D., Kirsch, H. L., Wortmann, R. L. & Pillinger, M. H. The causes of drug-induced muscle toxicity. Curr. Opin. Rheumatol. 26, 697–703 (2014).
Borges, I. B. P., Silva, M. G., Misse, R. G. & Shinjo, S. K. Lipid-lowering agent-triggered dermatomyositis and polymyositis: a case series and literature review. Rheumatol. Int. 38, 293–301 (2018).
Musset, L. et al. Anti-HMGCR antibodies as a biomarker for immune-mediated necrotizing myopathies: a history of statins and experience from a large international multi-center study. Autoimmun. Rev. 15, 983–993 (2016).
Orbach, H. & Tanay, A. Vaccines as a trigger for myopathies. Lupus 18, 1213–1216 (2009).
Svensson, J., Holmqvist, M., Lundberg, I. E. & Arkema, E. V. Infections and respiratory tract disease as risk factors for idiopathic inflammatory myopathies: a population-based case–control study. Ann. Rheum. Dis. 76, 1803–1808 (2017).
Lyon, M. G., Bloch, D. A., Hollak, B. & Fries, J. F. Predisposing factors in polymyositis-dermatomyositis: results of a nationwide survey. J. Rheumatol. 16, 1218–1224 (1989).
Chinoy, H. et al. Interaction of HLA-DRB1*03 and smoking for the development of anti-Jo-1 antibodies in adult idiopathic inflammatory myopathies: a European-wide case study. Ann. Rheum. Dis. 71, 961–965 (2012).
Lilleker, J. B. et al. The EuroMyositis registry: an international collaborative tool to facilitate myositis research. Ann. Rheum. Dis. 76, 862–868 (2017).
Koch, M. J., Brody, J. A. & Gillespie, M. M. Childhood polymyositis: a case-control study. Am. J. Epidemiol. 104, 627–631 (1976).
Orione, M. A. et al. Risk factors for juvenile dermatomyositis: exposure to tobacco and air pollutants during pregnancy. Arthritis Care Res. 66, 1571–1575 (2014).
Oddis, C. V., Conte, C. G., Steen, V. D. & Medsger, T. A. Jr. Incidence of polymyositis-dermatomyositis: a 20-year study of hospital diagnosed cases in Allegheny County, PA 1963–1982. J. Rheumatol. 17, 1329–1334 (1990).
Jacobson, D. L., Gange, S. J., Rose, N. R. & Graham, N. M. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin. Immunol. Immunopathol. 84, 223–243 (1997).
Goebels, N. et al. Differential expression of perforin in muscle-infiltrating T cells in polymyositis and dermato-myositis. J. Clin. Invest. 97, 2905–2910 (1996).
Orimo, S. et al. Immunohistochemical analysis of perforin and granzyme A in inflammatory myopathies. Neuromuscul. Disord. 4, 219–226 (1994).
Greenberg, S. A., Pinkus, J. L., Amato, A. A., Kristensen, T. & Dorfman, D. M. Association of inclusion body myositis with T cell large granular lymphocytic leukaemia. Brain 139, 1348–1360 (2016).
Hohlfeld, R., Engel, A. G., Ii, K. & Harper, M. C. Polymyositis mediated by T lymphocytes that express the gamma/delta receptor. N. Engl. J. Med. 324, 877–881 (1991).
Wiendl, H. et al. An autoreactive γδ TCR derived from a polymyositis lesion. J. Immunol. 169, 515–521 (2002).
Bruder, J. et al. Target specificity of an autoreactive pathogenic human γδ-T cell receptor in myositis. J. Biol. Chem. 287, 20986–20995 (2012).
Yamashita, T. et al. A case of myositis associated with clonal expansion of γδ T cells in peripheral blood and bone marrow [Japanese]. Rinsho Shinkeigaku 52, 227–233 (2012).
Fasth, A. E. et al. T cell infiltrates in the muscles of patients with dermatomyositis and polymyositis are dominated by CD28null T cells. J. Immunol. 183, 4792–4799 (2009).
Pandya, J. M. et al. Expanded T cell receptor Vβ-restricted T cells from patients with sporadic inclusion body myositis are proinflammatory and cytotoxic CD28null T cells. Arthritis Rheum. 62, 3457–3466 (2010).
Pandya, J. M. et al. CD4+ and CD8+ CD28null T cells are cytotoxic to autologous muscle cells in patients with polymyositis. Arthritis Rheumatol. 68, 2016–2026 (2016).
Waschbisch, A., Schwab, N., Ruck, T., Stenner, M. P. & Wiendl, H. FOXP3+ T regulatory cells in idiopathic inflammatory myopathies. J. Neuroimmunol. 225, 137–142 (2010).
Vercoulen, Y. et al. Increased presence of FOXP3+ regulatory T cells in inflamed muscle of patients with active juvenile dermatomyositis compared to peripheral blood. PLoS ONE 9, e105353 (2014).
Burzyn, D. et al. A special population of regulatory T cells potentiates muscle repair. Cell 155, 1282–1295 (2013).
Greenberg, S. A. et al. Plasma cells in muscle in inclusion body myositis and polymyositis. Neurology 65, 1782–1787 (2005).
Greenberg, S. A. et al. Molecular profiles of inflammatory myopathies. Neurology 59, 1170–1182 (2002).
Bradshaw, E. M. et al. A local antigen-driven humoral response is present in the inflammatory myopathies. J. Immunol. 178, 547–556 (2007).
Gunawardena, H., Betteridge, Z. E. & McHugh, N. J. Myositis-specific autoantibodies: their clinical and pathogenic significance in disease expression. Rheumatology 48, 607–612 (2009).
Betteridge, Z. & McHugh, N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J. Intern. Med. 280, 8–23 (2016).
Aggarwal, R. et al. Autoantibody levels in myositis patients correlate with clinical response during B cell depletion with rituximab. Rheumatology 55, 991–999 (2016).
Katsumata, Y. et al. Species-specific immune responses generated by histidyl-tRNA synthetase immunization are associated with muscle and lung inflammation. J. Autoimmun. 29, 174–186 (2007).
Howard, O. M. et al. Histidyl-tRNA synthetase and asparaginyl-tRNA synthetase, autoantigens in myositis, activate chemokine receptors on T lymphocytes and immature dendritic cells. J. Exp. Med. 196, 781–791 (2002).
Merad, M., Sathe, P., Helft, J., Miller, J. & Mortha, A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 31, 563–604 (2013).
Greenberg, S. A., Pinkus, G. S., Amato, A. A. & Pinkus, J. L. Myeloid dendritic cells in inclusion-body myositis and polymyositis. Muscle Nerve 35, 17–23 (2007).
Gendek-Kubiak, H. & Gendek, E. G. Fascin-expressing dendritic cells dominate in polymyositis and dermatomyositis. J. Rheumatol. 40, 186–191 (2013).
Nagaraju, K. et al. Endothelial cell activation and neovascularization are prominent in dermatomyositis. J. Autoimmune Dis. 3, 2 (2006).
Maddur, M. S. et al. Contribution of myeloid dendritic cells to type I interferon-induced cytokines and chemokines: comment on the article by Bilgic et al. Arthritis Rheum. 62, 2181–2182 (2010).
Chung, T., Christopher-Stine, L., Paik, J. J., Corse, A. & Mammen, A. L. The composition of cellular infiltrates in anti-HMG-CoA reductase-associated myopathy. Muscle Nerve 52, 189–195 (2015).
Rinnenthal, J. L. et al. Inflammatory myopathy with abundant macrophages (IMAM): the immunology revisited. Neuromuscul. Disord. 24, 151–155 (2014).
Liu, X. et al. Macrophage depletion impairs skeletal muscle regeneration: The roles of regulatory factors for muscle regeneration. Cell Biol. Int. 41, 228–238 (2017).
Rigamonti, E., Zordan, P., Sciorati, C., Rovere-Querini, P. & Brunelli, S. Macrophage plasticity in skeletal muscle repair. Biomed. Res. Int. 2014, 560629 (2014).
Tournadre, A., Lenief, V., Eljaafari, A. & Miossec, P. Immature muscle precursors are a source of interferon-β in myositis: role of Toll-like receptor 3 activation and contribution to HLA class I up-regulation. Arthritis Rheum. 64, 533–541 (2012).
Tournadre, A., Lenief, V. & Miossec, P. Expression of Toll-like receptor 3 and Toll-like receptor 7 in muscle is characteristic of inflammatory myopathy and is differentially regulated by Th1 and Th17 cytokines. Arthritis Rheum. 62, 2144–2151 (2010).
Cappelletti, C. et al. Type I interferon and Toll-like receptor expression characterizes inflammatory myopathies. Neurology 76, 2079–2088 (2011).
Moran, E. M. & Mastaglia, F. L. Cytokines in immune-mediated inflammatory myopathies: cellular sources, multiple actions and therapeutic implications. Clin. Exp. Immunol. 178, 405–415 (2014).
Rayavarapu, S., Coley, W., Kinder, T. B. & Nagaraju, K. Idiopathic inflammatory myopathies: pathogenic mechanisms of muscle weakness. Skelet. Muscle 3, 13 (2013).
De Paepe, B., Creus, K. K. & De Bleecker, J. L. Role of cytokines and chemokines in idiopathic inflammatory myopathies. Curr. Opin. Rheumatol. 21, 610–616 (2009).
Greenberg, S. A. et al. Interferon-α/β-mediated innate immune mechanisms in dermatomyositis. Ann. Neurol. 57, 664–678 (2005).
Salajegheh, M. et al. Interferon-stimulated gene 15 (ISG15) conjugates proteins in dermatomyositis muscle with perifascicular atrophy. Ann. Neurol. 67, 53–63 (2010).
Liao, A. P. et al. Interferon β is associated with type 1 interferon-inducible gene expression in dermatomyositis. Ann. Rheum. Dis. 70, 831–836 (2011).
Greenberg, S. A. Dermatomyositis and type 1 interferons. Curr. Rheumatol. Rep. 12, 198–203 (2010).
Baechler, E. C. et al. An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol. Med. 13, 59–68 (2007).
Greenberg, S. A. et al. Relationship between disease activity and type 1 interferon- and other cytokine-inducible gene expression in blood in dermatomyositis and polymyositis. Genes Immun. 13, 207–213 (2012).
Walsh, R. J. et al. Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum. 56, 3784–3792 (2007).
Krol, P. et al. Serum levels of interferon α do not correlate with disease activity in patients with dermatomyositis/polymyositis. Ann. Rheum. Dis. 70, 879–880 (2011).
Hervas-Stubbs, S. et al. Direct effects of type I interferons on cells of the immune system. Clin. Cancer Res. 17, 2619–2627 (2011).
Meyer, A. et al. IFN-β-induced reactive oxygen species and mitochondrial damage contribute to muscle impairment and inflammation maintenance in dermatomyositis. Acta Neuropathol. 134, 655–666 (2017).
Akeno, N. et al. IFN-α mediates the development of autoimmunity both by direct tissue toxicity and through immune cell recruitment mechanisms. J. Immunol. 186, 4693–4706 (2011).
Coomans de Brachene, A. et al. IFN-α induces a preferential long-lasting expression of MHC class I in human pancreatic beta cells. Diabetologia 61, 636–640 (2018).
Atta, M. S., Irving, W. L., Powell, R. J. & Todd, I. Enhanced expression of MHC class I molecules on cultured human thyroid follicular cells infected with reovirus through induction of type 1 interferons. Clin. Exp. Immunol. 101, 121–126 (1995).
Nagaraju, K. et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 52, 1824–1835 (2005).
Nagaraju, K. et al. Conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proc. Natl Acad. Sci. USA 97, 9209–9214 (2000).
Higgs, B. W. et al. A phase 1b clinical trial evaluating sifalimumab, an anti-IFN-α monoclonal antibody, shows target neutralisation of a type I IFN signature in blood of dermatomyositis and polymyositis patients. Ann. Rheum. Dis. 73, 256–262 (2014).
Manole, E., Bastian, A. E., Butoianu, N. & Goebel, H. H. Myositis non-inflammatory mechanisms: an up-dated review. J. Immunoassay Immunochem. 38, 115–126 (2017).
Coley, W., Rayavarapu, S. & Nagaraju, K. Role of non-immune mechanisms of muscle damage in idiopathic inflammatory myopathies. Arthritis Res. Ther. 14, 209 (2012).
Rayavarapu, S., Coley, W. & Nagaraju, K. Endoplasmic reticulum stress in skeletal muscle homeostasis and disease. Curr. Rheumatol. Rep. 14, 238–243 (2012).
Vitadello, M., Doria, A., Tarricone, E., Ghirardello, A. & Gorza, L. Myofiber stress-response in myositis: parallel investigations on patients and experimental animal models of muscle regeneration and systemic inflammation. Arthritis Res. Ther. 12, R52 (2010).
Vattemi, G., Engel, W. K., McFerrin, J. & Askanas, V. Endoplasmic reticulum stress and unfolded protein response in inclusion body myositis muscle. Am. J. Pathol. 164, 1–7 (2004).
Lightfoot, A. P., Nagaraju, K., McArdle, A. & Cooper, R. G. Understanding the origin of non-immune cell-mediated weakness in the idiopathic inflammatory myopathies – potential role of ER stress pathways. Curr. Opin. Rheumatol. 27, 580–585 (2015).
Monici, M. C., Aguennouz, M., Mazzeo, A., Messina, C. & Vita, G. Activation of nuclear factor-κB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology 60, 993–997 (2003).
De Paepe, B. et al. Activation of osmolyte pathways in inflammatory myopathy and Duchenne muscular dystrophy points to osmoregulation as a contributing pathogenic mechanism. Lab. Invest. 96, 872–884 (2016).
Bhattarai, S. et al. The immunoproteasomes are key to regulate myokines and MHC class I expression in idiopathic inflammatory myopathies. J. Autoimmun. 75, 118–129 (2016).
Yin, X., Han, G. C., Jiang, X. W., Shi, Q. & Pu, C. Q. Increased expression of the NOD-like receptor family, pyrin domain containing 3 inflammasome in dermatomyositis and polymyositis is a potential contributor to their pathogenesis. Chin. Med. J. 129, 1047–1052 (2016).
Bae, H. R. et al. β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget 7, 66444–66454 (2016).
Wanchu, A., Khullar, M., Sud, A. & Bambery, P. Nitric oxide production is increased in patients with inflammatory myositis. Nitric Oxide 3, 454–458 (1999).
Tews, D. S. & Goebel, H. H. Cell death and oxidative damage in inflammatory myopathies. Clin. Immunol. Immunopathol. 87, 240–247 (1998).
Lambert, K. et al. Grape polyphenols supplementation reduces muscle atrophy in a mouse model of chronic inflammation. Nutrition 31, 1275–1283 (2015).
Alger, H. M. et al. The role of TRAIL in mediating autophagy in myositis skeletal muscle: a potential nonimmune mechanism of muscle damage. Arthritis Rheum. 63, 3448–3457 (2011).
De Paepe, B., Creus, K. K., Martin, J. J., Weis, J. & De Bleecker, J. L. A dual role for HSP90 and HSP70 in the inflammatory myopathies: from muscle fiber protection to active invasion by macrophages. Ann. NY Acad. Sci. 1173, 463–469 (2009).
Grundtman, C. et al. Effects of HMGB1 on in vitro responses of isolated muscle fibers and functional aspects in skeletal muscles of idiopathic inflammatory myopathies. FASEB J. 24, 570–578 (2010).
Ulfgren, A. K. et al. Down-regulation of the aberrant expression of the inflammation mediator high mobility group box chromosomal protein 1 in muscle tissue of patients with polymyositis and dermatomyositis treated with corticosteroids. Arthritis Rheum. 50, 1586–1594 (2004).
Zong, M. et al. TLR4 as receptor for HMGB1 induced muscle dysfunction in myositis. Ann. Rheum. Dis. 72, 1390–1399 (2013).
Harris, H. E., Andersson, U. & Pisetsky, D. S. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol. 8, 195–202 (2012).
Wan, Z. et al. TLR4-HMGB1 signaling pathway affects the inflammatory reaction of autoimmune myositis by regulating MHC-I. Int. Immunopharmacol. 41, 74–81 (2016).
Cseri, K. et al. HMGB1 expression and muscle regeneration in idiopathic inflammatory myopathies and degenerative joint diseases. J. Muscle Res. Cell. Motil. 36, 255–262 (2015).
Shu, X., Peng, Q., Lu, X. & Wang, G. HMGB1 may be a biomarker for predicting the outcome in patients with polymyositis /dermatomyositis with interstitial lung disease. PLoS ONE 11, e0161436 (2016).
Keller, C. W., Schmidt, J. & Lunemann, J. D. Immune and myodegenerative pathomechanisms in inclusion body myositis. Ann. Clin. Transl Neurol. 4, 422–445 (2017).
Vattemi, G. et al. Amyloid-β42 is preferentially accumulated in muscle fibers of patients with sporadic inclusion-body myositis. Acta Neuropathol. 117, 569–574 (2009).
Askanas, V., Engel, W. K. & Nogalska, A. Sporadic inclusion-body myositis: a degenerative muscle disease associated with aging, impaired muscle protein homeostasis and abnormal mitophagy. Biochim. Biophys. Acta 1852, 633–643 (2015).
Askanas, V. & King Engel, W. Update on neuromuscular diseases: pathology and molecular pathogenesis. Biochim. Biophys. Acta 1852, 561–562 (2015).
Muth, I. E., Barthel, K., Bahr, M., Dalakas, M. C. & Schmidt, J. Pro-inflammatory cell stress in sporadic inclusion body myositis: overexpression of αB-crystallin is associated with amyloid precursor protein and accumulation of α-amyloid. J. Neurol. Neurosurg. Psychiatry 80, 1344–1349 (2009).
Wojcik, S., Engel, W. K., McFerrin, J., Paciello, O. & Askanas, V. AβPP-overexpression and proteasome inhibition increase αB-crystallin in cultured human muscle: relevance to inclusion-body myositis. Neuromuscul. Disord. 16, 839–844 (2006).
Banwell, B. L. & Engel, A. G. αB-crystallin immunolocalization yields new insights into inclusion body myositis. Neurology 54, 1033–1041 (2000).
Wang, P., Wander, C. M., Yuan, C. X., Bereman, M. S. & Cohen, T. J. Acetylation-induced TDP-43 pathology is suppressed by an HSF1-dependent chaperone program. Nat. Commun. 8, 82 (2017).
Ahmed, M. et al. Targeting protein homeostasis in sporadic inclusion body myositis. Sci. Transl. Med. 8, 331ra41 (2016).
Nogalska, A., D'Agostino, C., Terracciano, C., Engel, W. K. & Askanas, V. Impaired autophagy in sporadic inclusion-body myositis and in endoplasmic reticulum stress-provoked cultured human muscle fibers. Am. J. Pathol. 177, 1377–1387 (2010).
Cacciottolo, M., Nogalska, A., D'Agostino, C., Engel, W. K. & Askanas, V. Chaperone-mediated autophagy components are upregulated in sporadic inclusion-body myositis muscle fibres. Neuropathol. Appl. Neurobiol. 39, 750–761 (2013).
Lunemann, J. D. et al. Beta-amyloid is a substrate of autophagy in sporadic inclusion body myositis. Ann. Neurol. 61, 476–483 (2007).
Schmidt, K. et al. IL-1β-induced accumulation of amyloid: macroautophagy in skeletal muscle depends on ERK. Mediators Inflamm. 2017, 5470831 (2017).
Nakano, S., Oki, M. & Kusaka, H. The role of p62/SQSTM1 in sporadic inclusion body myositis. Neuromuscul. Disord. 27, 363–369 (2017).
Nicot, A. S. et al. Phosphorylation of NBR1 by GSK3 modulates protein aggregation. Autophagy 10, 1036–1053 (2014).
D'Agostino, C., Nogalska, A., Cacciottolo, M., King Engel, W. & Askanas, V. Abnormalities of NBR1, a novel autophagy-associated protein, in muscle fibers of sporadic inclusion-body myositis. Acta Neuropathol. 122, 627 (2011).
Duleh, S., Wang, X., Komirenko, A. & Margeta, M. Activation of the Keap1/Nrf2 stress response pathway in autophagic vacuolar myopathies. Acta Neuropathol. Commun. 4, 115 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02481453 (2017).
Oldfors, A. et al. Mitochondrial abnormalities in inclusion-body myositis. Neurology 66, S49–S55 (2006).
Catalán-García, M. et al. Mitochondrial DNA disturbances and deregulated expression of oxidative phosphorylation and mitochondrial fusion proteins in sporadic inclusion body myositis. Clin. Sci. 130, 1741–1751 (2016).
Rygiel, K. A. et al. Mitochondrial and inflammatory changes in sporadic inclusion body myositis. Neuropathol. Appl. Neurobiol. 41, 288–303 (2015).
Joshi, P. R. et al. Functional relevance of mitochondrial abnormalities in sporadic inclusion body myositis. J. Clin. Neurosci. 21, 1959–1963 (2014).
Boncompagni, S. et al. Mitochondrial dysfunction in skeletal muscle of amyloid precursor protein-overexpressing mice. J. Biol. Chem. 287, 20534–20544 (2012).
Askanas, V. et al. Transfer of beta-amyloid precursor protein gene using adenovirus vector causes mitochondrial abnormalities in cultured normal human muscle. Proc. Natl Acad. Sci. USA 93, 1314–1319 (1996).
Schmidt, J. et al. Nitric oxide stress in sporadic inclusion body myositis muscle fibres: inhibition of inducible nitric oxide synthase prevents interleukin-1β-induced accumulation of β-amyloid and cell death. Brain 135, 1102–1114 (2012).
Baron, P. et al. Synergistic effect of β-amyloid protein and interferon gamma on nitric oxide production by C2C12 muscle cells. Brain 123, 374–379 (2000).
Rayavarapu, S. et al. Activation of the ubiquitin proteasome pathway in a mouse model of inflammatory myopathy: a potential therapeutic target. Arthritis Rheum. 65, 3248–3258 (2013).
Rider, L. G., Danko, K. & Miller, F. W. Myositis registries and biorepositories: powerful tools to advance clinical, epidemiologic and pathogenic research. Curr. Opin. Rheumatol. 26, 724–741 (2014).
Molberg, O. & Dobloug, C. Epidemiology of sporadic inclusion body myositis. Curr. Opin. Rheumatol. 28, 657–660 (2016).
Helmers, S. B. et al. Inflammatory lung disease a potential risk factor for onset of idiopathic inflammatory myopathies: results from a pilot study. RMD Open 2, e000342 (2016).
Mamyrova, G., Rider, L. G., Haagenson, L., Wong, S. & Brown, K. E. Parvovirus B19 and onset of juvenile dermatomyositis. JAMA 294, 2170–2171 (2005).
Acknowledgements
The authors thank L. Rider, I. Lundberg, A. Mammen and C. Parks for many useful discussions of concepts in this area and for helpful comments on the manuscript, and are grateful to L. Maroski for her technical assistance. The authors' work is supported in part by the Intramural Research Program of the NIH National Institute of Environmental Health Sciences. K.N. is supported by the NIH (1R21AI128248-01, K26OD011171), The Myositis Association and the US Department of Defense (W81XWH-11-1-0809 (K.N. and F.W.M.), W81XWH-11-1-0782 (K.N.). J.L. is supported by the Medical Research Council, UK (MR/N003322/1) and The Myositis Association.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussions of the content, wrote the article and contributed equally to reviewing and/or editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Rimmed vacuoles
-
Spaces within the cytoplasm of a muscle cell with a purplish staining rim on trichrome staining.
- Functional annotation
-
Characterization of the function assigned to each gene product or genetic variant.
- Expression quantitative trait loci
-
(eQTLs). Genomic loci that regulate gene expression.
- Macroautophagy
-
A process in which cellular contents are degraded by lysosomes or vacuoles and recycled.
Rights and permissions
About this article
Cite this article
Miller, F., Lamb, J., Schmidt, J. et al. Risk factors and disease mechanisms in myositis. Nat Rev Rheumatol 14, 255–268 (2018). https://doi.org/10.1038/nrrheum.2018.48
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2018.48
This article is cited by
-
Immune cell profiles of idiopathic inflammatory myopathy patients expressed anti-aminoacyl tRNA synthetase or anti-melanoma differentiation-associated gene 5 autoantibodies
BMC Immunology (2023)
-
Myositis: von der Diagnose zur Therapie
Der Nervenarzt (2023)
-
Plasma proteomic profiling reveals KRT19 could be a potential biomarker in patients with anti-MDA5+ dermatomyositis
Clinical Rheumatology (2023)
-
Increased serum soluble interleukin-2 receptor levels in dermatomyositis are associated with Th17/Treg immune imbalance
Clinical and Experimental Medicine (2023)
-
The spectrum of idiopathic inflammatory myopathies in Western Australia: epidemiological characteristics and mortality over time
Rheumatology International (2023)