Key Points
-
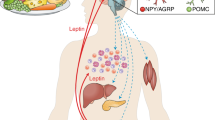
Leptin is an adipokine produced predominantly by adipose tissue, but also expressed in the articulation by chondrocytes and synoviocytes, and by immune cells
-
As a consequence of its dual role as an anorexigenic and a proinflammatory factor, leptin is now considered a link between the neuroendocrine and immune systems
-
Leptin participates in innate immunity by inhibiting natural killer cells and by inducing proliferation and activation of monocytes
-
Leptin signalling can also regulate adaptive immunity by activating T-cell proliferation and responsiveness, and by stimulating B-cell proliferation and cytokine production
-
Leptin can exert its proinflammatory and pro-catabolic actions on cartilage, leading to articular degeneration characteristic of osteoarthritis
Abstract
Leptin is one of the most relevant factors secreted by adipose tissue and the forerunner of a class of molecules collectively called adipokines. Initially discovered in 1994, its crucial role as a central regulator in energy homeostasis has been largely described during the past 20 years. Once secreted into the circulation, leptin reaches the central and peripheral nervous systems and acts by binding and activating the long form of leptin receptor (LEPR), regulating appetite and food intake, bone mass, basal metabolism, reproductive function and insulin secretion, among other processes. Research on the regulation of different adipose tissues has provided important insights into the intricate network that links nutrition, metabolism and immune homeostasis. The neuroendocrine and immune systems communicate bi-directionally through common ligands and receptors during stress responses and inflammation, and control cellular immune responses in several pathological situations including immune-inflammatory rheumatic diseases. This Review discusses the latest findings regarding the role of leptin in the immune system and metabolism, with particular emphasis on its effect on autoimmune and/or inflammatory rheumatic diseases, such as rheumatoid arthritis and osteoarthritis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994). This is a seminal paper that described for the first time the existence of leptin in human and mice.
Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 115, 911–919 (2005).
Hotamisligil, G. S., Shargill, N. S. & Spiegelman, B. M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91 (1993). This paper demonstrated for the first time that white adipose tissue is an active metabolic tissue rather than a mere fat depot.
Fantuzzi, G. & Faggioni, R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J. Leukoc. Biol. 68, 437–446 (2000).
Lago, F., Dieguez, C., Gómez-Reino, J. & Gualillo, O. Adipokines as emerging mediators of immune response and inflammation. Nat. Clin. Pract. Rheumatol. 3, 716–724 (2007).
Otero, M., Lago, R., Lago, F., Reino, J. J. G. & Gualillo, O. Signalling pathway involved in nitric oxide synthase type II activation in chondrocytes: synergistic effect of leptin with interleukin-1. Arthritis Res. Ther. 7, 581–591 (2005).
Tilg, H. & Moschen, A. R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 6, 772–783 (2006).
Lago, F., Gómez, R., Gómez-Reino, J. J., Dieguez, C. & Gualillo, O. Adipokines as novel modulators of lipid metabolism. Trends Biochem. Sci. 34, 500–510 (2009).
Lago, F., Dieguez, C., Gómez-Reino, J. & Gualillo, O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 18, 313–325 (2007).
Frühbeck, G. Intracellular signalling pathways activated by leptin. Biochem. J. 393, 7–20 (2006).
Procaccini, C., Jirillo, E. & Matarese, G. Leptin as an immunomodulator. Mol. Aspects Med. 33, 35–45 (2012).
Carlton, E. D., Demas, G. E. & French, S. S. Leptin, a neuroendocrine mediator of immune responses, inflammation, and sickness behaviors. Horm. Behav. 62, 272–279 (2012).
La Cava, A. & Matarese, G. The weight of leptin in immunity. Nat. Rev. Immunol. 4, 371–379 (2004). This review summarizes the most relevant aspects of leptin in the regulation of immune response.
Rosenbaum, M. & Leibel, R. L. 20 years of leptin: role of leptin in energy homeostasis in humans. J. Endocrinol. 223, T83–T96 (2014).
Di Carlo, C., Tommaselli, G. A. & Nappi, C. Effects of sex steroid hormones and menopause on serum leptin concentrations. Gynecol. Endocrinol. 16, 479–491 (2002).
Gualillo, O., Eiras, S., Lago, F., Diéguez, C. & Casanueva, F. F. Elevated serum leptin concentrations induced by experimental acute inflammation. Life Sci. 67, 2433–2441 (2000). This is the first study demonstrating the leptin increase in acute inflammation.
Behnes, M. et al. Alterations of leptin in the course of inflammation and severe sepsis. BMC Infect. Dis. 12, 217 (2012).
Feijóo-Bandín, S. et al. 20 years of leptin: role of leptin in cardiomyocyte physiology and physiopathology. Life Sci. 140, 10–18 (2015).
Chou, S. H. & Mantzoros, C. 20 years of leptin: role of leptin in human reproductive disorders. J. Endocrinol. 223, T49–62 (2014).
Conde, J. et al. An update on leptin as immunomodulator. Expert Rev. Clin. Immunol. 10, 1165–1170 (2014).
Scotece, M. et al. Adipokines as drug targets in joint and bone disease. Drug Discov. Today http://dx.doi.org/10.1016/j.drudis.2013.07.012 (2013).
Gómez, R. et al. What's new in our understanding of the role of adipokines in rheumatic diseases? Nat. Rev. Rheumatol. 7, 528–536 (2011).
Edwards, C. et al. The effects of bariatric surgery weight loss on knee pain in patients with osteoarthritis of the knee. Arthritis 2012, 504189 (2012).
Wluka, A. E., Lombard, C. B. & Cicuttini, F. M. Tackling obesity in knee osteoarthritis. Nat. Rev. Rheumatol. 9, 225–235 (2013).
Yusuf, E. et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann. Rheum. Dis. 69, 761–765 (2010).
Grotle, M., Hagen, K. B., Natvig, B., Dahl, F. A. & Kvien, T. K. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet. Disord. 9, 132 (2008).
Sakkas, L. I. et al. T cells and T-cell cytokine transcripts in the synovial membrane in patients with osteoarthritis. Clin. Diagn. Lab. Immunol. 5, 430–437 (1998).
Nakamura, H., Yoshino, S., Kato, T., Tsuruha, J. & Nishioka, K. T-cell mediated inflammatory pathway in osteoarthritis. Osteoarthritis Cartilage 7, 401–402 (1999).
Ishii, H. et al. Characterization of infiltrating T cells and Th1/Th2-type cytokines in the synovium of patients with osteoarthritis. Osteoarthritis Cartilage 10, 277–281 (2002).
Tian, Z., Sun, R., Wei, H. & Gao, B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem. Biophys. Res. Commun. 298, 297–302 (2002). This study demonstrated the involvement of leptin in natural killer cell activity.
Zhao, Y., Sun, R., You, L., Gao, C. & Tian, Z. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem. Biophys. Res. Commun. 300, 247–252 (2003).
Nave, H. et al. Resistance of Janus kinase-2 dependent leptin signaling in natural killer (NK) cells: a novel mechanism of NK cell dysfunction in diet-induced obesity. Endocrinology 149, 3370–3378 (2008).
Lo, C. K. C. et al. Leptin signaling protects NK cells from apoptosis during development in mouse bone marrow. Cell. Mol. Immunol. 6, 353–360 (2009).
Laue, T. et al. Altered NK cell function in obese healthy humans. BMC Obes. 2, 1 (2015).
Wrann, C. D. et al. Short-term and long-term leptin exposure differentially affect human natural killer cell immune functions. Am. J. Physiol. Endocrinol. Metab. 302, E108–E116 (2012).
Lamas, B. et al. Leptin modulates dose-dependently the metabolic and cytolytic activities of NK-92 cells. J. Cell. Physiol. 228, 1202–1209 (2013).
Jahn, J. et al. Decreased NK cell functions in obesity can be reactivated by fat mass reduction. Obes. (Silver Spring). 23, 2233–2241 (2015).
Caldefie-Chezet, F., Poulin, A., Tridon, A., Sion, B. & Vasson, M. P. Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? J. Leukoc. Biol. 69, 414–418 (2001).
Zarkesh-Esfahani, H. et al. Leptin indirectly activates human neutrophils via induction of TNF-α. J. Immunol. 172, 1809–1814 (2004).
Bjorbaek, C., Uotani, S., da Silva, B. & Flier, J. S. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J. Biol. Chem. 272, 32686–32695 (1997).
Caldefie-Chezet, F., Poulin, A. & Vasson, M. P. Leptin regulates functional capacities of polymorphonuclear neutrophils. Free Radic. Res. 37, 809–814 (2003).
Montecucco, F. et al. Induction of neutrophil chemotaxis by leptin: crucial role for p38 and Src kinases. Ann. NY Acad. Sci. 1069, 463–471 (2006).
Bruno, A., Conus, S., Schmid, I. & Simon, H.-U. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J. Immunol. 174, 8090–8096 (2005).
Sun, Z., Dragon, S., Becker, A. & Gounni, A. S. Leptin inhibits neutrophil apoptosis in children via ERK/NF-κB-dependent pathways. PLoS ONE 8, e55249 (2013).
Kamp, V. M. et al. Physiological concentrations of leptin do not affect human neutrophils. PLoS ONE 8, e73170 (2013).
do Carmo, L. S. et al. A high-fat diet increases interleukin-3 and granulocyte colony-stimulating factor production by bone marrow cells and triggers bone marrow hyperplasia and neutrophilia in Wistar rats. Exp. Biol. Med. (Maywood). 238, 375–384 (2013).
Brotfain, E. et al. Neutrophil functions in morbidly obese subjects. Clin. Exp. Immunol. 181, 156–163 (2015).
Conus, S., Bruno, A. & Simon, H.-U. Leptin is an eosinophil survival factor. J. Allergy Clin. Immunol. 116, 1228–1234 (2005).
Kato, H. et al. Leptin has a priming effect on eotaxin-induced human eosinophil chemotaxis. Int. Arch. Allergy Immunol. 155, 335–344 (2011).
Wong, C. K., Cheung, P. F.-Y. & Lam, C. W. K. Leptin-mediated cytokine release and migration of eosinophils: implications for immunopathophysiology of allergic inflammation. Eur. J. Immunol. 37, 2337–2348 (2007).
Suzukawa, M. et al. Leptin enhances survival and induces migration, degranulation, and cytokine synthesis of human basophils. J. Immunol. 186, 5254–5260 (2011).
Tsiotra, P. C., Pappa, V., Raptis, S. A. & Tsigos, C. Expression of the long and short leptin receptor isoforms in peripheral blood mononuclear cells: implications for leptin's actions. Metabolism. 49, 1537–1541 (2000).
O'Rourke, L., Yeaman, S. J. & Shepherd, P. R. Insulin and leptin acutely regulate cholesterol ester metabolism in macrophages by novel signaling pathways. Diabetes 50, 955–961 (2001).
Santos-Alvarez, J., Goberna, R. & Sánchez-Margalet, V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell. Immunol. 194, 6–11 (1999). This is the first evidence of the effect of leptin in monocyte proliferation and activation.
Conde, J. et al. At the crossroad between immunity and metabolism: focus on leptin. Expert Rev. Clin. Immunol. 6, 801–808 (2010).
Gutierrez, D. A. & Hasty, A. H. Haematopoietic leptin receptor deficiency does not affect macrophage accumulation in adipose tissue or systemic insulin sensitivity. J. Endocrinol. 212, 343–351 (2012).
Tsiotra, P. C., Boutati, E., Dimitriadis, G. & Raptis, S. A. High insulin and leptin increase resistin and inflammatory cytokine production from human mononuclear cells. Biomed. Res. Int. 2013, 487081 (2013).
Jitprasertwong, P., Jaedicke, K. M., Nile, C. J., Preshaw, P. M. & Taylor, J. J. Leptin enhances the secretion of interleukin (IL)-18, but not IL-1β, from human monocytes via activation of caspase-1. Cytokine 65, 222–230 (2014).
Gómez, R., Villalvilla, A., Largo, R., Gualillo, O. & Herrero-Beaumont, G. TLR4 signalling in osteoarthritis—finding targets for candidate DMOADs. Nat. Rev. Rheumatol. 11, 159–170 (2015).
Jaedicke, K. M. et al. Leptin up-regulates TLR2 in human monocytes. J. Leukoc. Biol. 93, 561–571 (2013).
Acedo, S. C., Gambero, S., Cunha, F. G. P., Lorand-Metze, I. & Gambero, A. Participation of leptin in the determination of the macrophage phenotype: an additional role in adipocyte and macrophage crosstalk. In Vitro Cell. Dev. Biol. Anim. 49, 473–478 (2013).
Luan, B. et al. Leptin-mediated increases in catecholamine signaling reduce adipose tissue inflammation via activation of macrophage HDAC4. Cell Metab. 19, 1058–1065 (2014).
Zhou, Y. et al. Leptin deficiency shifts mast cells toward anti-inflammatory actions and protects mice from obesity and diabetes by polarizing M2 macrophages. Cell Metab. 22, 1045–1058 (2015).
Munoz, L. E. et al. Apoptosis in the pathogenesis of systemic lupus erythematosus. Lupus 17, 371–375 (2008).
Amarilyo, G., Iikuni, N., Liu, A., Matarese, G. & La Cava, A. Leptin enhances availability of apoptotic cell-derived self-antigen in systemic lupus erythematosus. PLoS ONE 9, e112826 (2014).
Okano, T. et al. Hyperleptinemia suppresses aggravation of arthritis of collagen-antibody-induced arthritis in mice. J. Orthop. Sci. 20, 1106–1113 (2015).
Voloshyna, I., Mounessa, J., Carsons, S. E. & Reiss, A. B. Effect of inhibition of interleukin-12/23 by ustekinumab on the expression of leptin and leptin receptor in human THP-1 macrophages. Clin. Exp. Dermatol. 41, 308–311 (2016).
Dib, L. H., Ortega, M. T., Melgarejo, T. & Chapes, S. K. Establishment and characterization of DB-1: a leptin receptor-deficient murine macrophage cell line. Cytotechnology http://dx.doi.org/10.1007/s10616-015-9843-3 (2015).
Mattioli, B., Straface, E., Quaranta, M. G., Giordani, L. & Viora, M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J. Immunol. 174, 6820–6828 (2005).
Lam, Q. L. K., Liu, S., Cao, X. & Lu, L. Involvement of leptin signaling in the survival and maturation of bone marrow-derived dendritic cells. Eur. J. Immunol. 36, 3118–3130 (2006).
Mattioli, B., Giordani, L., Quaranta, M. G. & Viora, M. Leptin exerts an anti-apoptotic effect on human dendritic cells via the PI3K-Akt signaling pathway. FEBS Lett. 583, 1102–1106 (2009).
Al-Hassi, H. O. et al. A mechanistic role for leptin in human dendritic cell migration: differences between ileum and colon in health and Crohn's disease. Mucosal Immunol. 6, 751–761 (2013).
Mattioli, B. et al. Leptin as an immunological adjuvant: enhanced migratory and CD8+ T cell stimulatory capacity of human dendritic cells exposed to leptin. FASEB J. 22, 2012–2022 (2008).
Moraes-Vieira, P. M. M. et al. Leptin deficiency impairs maturation of dendritic cells and enhances induction of regulatory T and Th17 cells. Eur. J. Immunol. 44, 794–806 (2014).
Ramirez, O. & Garza, K. M. Leptin deficiency in vivo enhances the ability of splenic dendritic cells to activate T cells. Int. Immunol. 26, 627–636 (2014).
Kimura, M. et al. T lymphopenia in obese diabetic (db/db) mice is non-selective and thymus independent. Life Sci. 62, 1243–1250 (1998).
Lord, G. M. et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394, 897–901 (1998). This is the first report demonstrating the relationship between leptin and the adaptive immune system.
Sanchez-Margalet, V. & Martin-Romero, C. Human leptin signaling in human peripheral blood mononuclear cells: activation of the JAK-STAT pathway. Cell. Immunol. 211, 30–36 (2001).
De Rosa, V. et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity 26, 241–255 (2007).
Farooqi, I. S. et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 110, 1093–1103 (2002).
Batra, A. et al. Leptin: a critical regulator of CD4+ T-cell polarization in vitro and in vivo. Endocrinology 151, 56–62 (2010).
Kim, S. Y. et al. Preferential effects of leptin on CD4 T cells in central and peripheral immune system are critically linked to the expression of leptin receptor. Biochem. Biophys. Res. Commun. 394, 562–568 (2010).
Matarese, G., Procaccini, C., De Rosa, V., Horvath, T. L. & La Cava, A. Regulatory T cells in obesity: the leptin connection. Trends Mol. Med. 16, 247–256 (2010).
Cassano, S. et al. Leptin modulates autophagy in human CD4+CD25− conventional T cells. Metabolism. 63, 1272–1279 (2014).
Procaccini, C. et al. Leptin-induced mTOR activation defines a specific molecular and transcriptional signature controlling CD4+ effector T cell responses. J. Immunol. 189, 2941–2953 (2012).
Ma, L. et al. Elevated serum leptin levels in patients with systemic lupus erythematosus. Pharmazie 70, 720–723 (2015).
Liu, Y., Yu, Y., Matarese, G. & La Cava, A. Cutting edge: Fasting-induced hypoleptinemia expands functional regulatory T cells in systemic lupus erythematosus. J. Immunol. 188, 2070–2073 (2012).
Fujita, Y. et al. Deficient leptin signaling ameliorates systemic lupus erythematosus lesions in MRL/Mp-Fas lpr mice. J. Immunol. 192, 979–984 (2014).
Margiotta, D. et al. Relationship between leptin and regulatory T cells in systemic lupus erythematosus: preliminary results. Eur. Rev. Med. Pharmacol. Sci. 20, 636–641 (2016).
Yu, Y. et al. Cutting edge: Leptin-induced RORγt expression in CD4+ T cells promotes Th17 responses in systemic lupus erythematosus. J. Immunol. 190, 3054–3058 (2013).
Deng, J. et al. Leptin exacerbates collagen-induced arthritis via enhancement of Th17 cell response. Arthritis Rheum. 64, 3564–3573 (2012).
Reis, B. S. et al. Leptin receptor signaling in T cells is required for Th17 differentiation. J. Immunol. 194, 5253–5260 (2015).
Busso, N. et al. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J. Immunol. 168, 875–882 (2002).
Bennett, B. D. et al. A role for leptin and its cognate receptor in hematopoiesis. Curr. Biol. 6, 1170–1180 (1996).
Claycombe, K., King, L. E. & Fraker, P. J. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc. Natl Acad. Sci. USA 105, 2017–2021 (2008).
Lam, Q. L. K., Wang, S., Ko, O. K. H., Kincade, P. W. & Lu, L. Leptin signaling maintains B-cell homeostasis via induction of Bcl-2 and Cyclin D1. Proc. Natl Acad. Sci. USA 107, 13812–13817 (2010).
Agrawal, S., Gollapudi, S., Su, H. & Gupta, S. Leptin activates human B cells to secrete TNF-α, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J. Clin. Immunol. 31, 472–478 (2011).
Gupta, S., Agrawal, S. & Gollapudi, S. Increased activation and cytokine secretion in B cells stimulated with leptin in aged humans. Immun. Ageing 10, 3 (2013).
Tanaka, M. et al. Role of central leptin signaling in the starvation-induced alteration of B-cell development. J. Neurosci. 31, 8373–8380 (2011).
Fujita, Y. et al. Prevention of fasting-mediated bone marrow atrophy by leptin administration. Cell. Immunol. 273, 52–58 (2012).
Frasca, D. et al. Obesity decreases B cell responses in young and elderly individuals. Obesity 24, 615–625 (2016).
Scotece, M. & Mobasheri, A. Leptin in osteoarthritis: focus on articular cartilage and chondrocytes. Life Sci. 140, 75–78 (2015).
de Boer, T. N. et al. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis Cartilage 20, 846–853 (2012).
Figenschau, Y., Knutsen, G., Shahazeydi, S., Johansen, O. & Sveinbjörnsson, B. Human articular chondrocytes express functional leptin receptors. Biochem. Biophys. Res. Commun. 287, 190–197 (2001).
Dumond, H. et al. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 48, 3118–3129 (2003).
Otero, M., Gomez Reino, J. J. & Gualillo, O. Synergistic induction of nitric oxide synthase type II: in vitro effect of leptin and interferon-gamma in human chondrocytes and ATDC5 chondrogenic cells. Arthritis Rheum. 48, 404–409 (2003). This is the first published evidence of the activity of leptin in the regulation of inflammatory response in human and mouse chondrocytes.
Conde, J. et al. Adipokines and osteoarthritis: novel molecules involved in the pathogenesis and progression of disease. Arthritis 2011, 1–8 (2011).
Toussirot, E., Streit, G. & Wendling, D. The contribution of adipose tissue and adipokines to inflammation in joint diseases. Curr. Med. Chem. 14, 1095–1100 (2007).
Hui, W. et al. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann. Rheum. Dis. 71, 455–462 (2012).
Bao, J. et al. Leptin plays a catabolic role on articular cartilage. Mol. Biol. Rep. 37, 3265–3272 (2010).
Gómez, R. et al. Adiponectin and leptin increase IL-8 production in human chondrocytes. Ann. Rheum. Dis. 70, 2052–2054 (2011).
Conde, J. et al. Adiponectin and leptin induce VCAM-1 expression in human and murine chondrocytes. PLoS ONE 7, e52533 (2012).
Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 15, 692–704 (2015).
Conde, J. et al. Expanding the adipokine network in cartilage: identification and regulation of novel factors in human and murine chondrocytes. Ann. Rheum. Dis. 70, 551–559 (2011). This article described for the first time the expression of a novel network of adipokines locally produced in human and mouse cartilage.
Conde, J. et al. Basic aspects of adipokines in bone metabolism. Clin. Rev. Bone Miner. Metab. 13, 11–19 (2015).
Findlay, D. M. & Atkins, G. J. Osteoblast-chondrocyte interactions in osteoarthritis. Curr. Osteoporos. Rep. 12, 127–134 (2014).
Mutabaruka, M.-S., Aoulad Aissa, M., Delalandre, A., Lavigne, M. & Lajeunesse, D. Local leptin production in osteoarthritis subchondral osteoblasts may be responsible for their abnormal phenotypic expression. Arthritis Res. Ther. 12, R20 (2010).
Ducy, P. et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100, 197–207 (2000).
Vuolteenaho, K., Koskinen, A., Moilanen, T. & Moilanen, E. Leptin levels are increased and its negative regulators, SOCS-3 and sOb-R are decreased in obese patients with osteoarthritis: a link between obesity and osteoarthritis. Ann. Rheum. Dis. 71, 1912–1913 (2012).
Koskinen, A., Vuolteenaho, K., Nieminen, R., Moilanen, T. & Moilanen, E. Leptin enhances MMP-1, MMP-3 and MMP-13 production in human osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in synovial fluid from OA patients. Clin. Exp. Rheumatol. 29, 57–64 (2011).
Scotece, M. et al. Adipokines induce pro-inflammatory factors in activated CD4+ T cells from osteoarthritis patients. J. Orthop. Res. http://dx.doi.org/10.1002/jor.23377 (2016).
Griffin, T. M., Huebner, J. L., Kraus, V. B. & Guilak, F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. 60, 2935–2944 (2009).
Massengale, M., Lu, B., Pan, J. J., Katz, J. N. & Solomon, D. H. Adipokine hormones and hand osteoarthritis: radiographic severity and pain. PLoS ONE 7, e47860 (2012).
Conde, J. et al. Differential expression of adipokines in infrapatellar fat pad (IPFP) and synovium of osteoarthritis patients and healthy individuals. Ann. Rheum. Dis. 73, 631–633 (2014). This article demonstrated that the infrapatellar fat pad is an active proinflammatory tissue secreting adipokines.
Zhao, X. et al. Leptin changes differentiation fate and induces senescence in chondrogenic progenitor cells. Cell Death Dis. 7, e2188 (2016).
Lee, S.-W., Park, M.-C., Park, Y.-B. & Lee, S.-K. Measurement of the serum leptin level could assist disease activity monitoring in rheumatoid arthritis. Rheumatol. Int. 27, 537–540 (2007).
Targonska-Stepniak, B., Majdan, M. & Dryglewska, M. Leptin serum levels in rheumatoid arthritis patients: relation to disease duration and activity. Rheumatol. Int. 28, 585–591 (2008).
Targonska-Stepniak, B., Dryglewska, M. & Majdan, M. Adiponectin and leptin serum concentrations in patients with rheumatoid arthritis. Rheumatol. Int. 30, 731–737 (2010).
Olama, S. M., Senna, M. K. & Elarman, M. Synovial/serum leptin ratio in rheumatoid arthritis: the association with activity and erosion. Rheumatol. Int. 32, 683–690 (2012).
Xibillé-Friedmann, D., Bustos-Bahena, C., Hernández-Góngora, S., Burgos-Vargas, R. & Montiel-Hernández, J. L. Two-year follow-up of plasma leptin and other cytokines in patients with rheumatoid arthritis. Ann. Rheum. Dis. 69, 930–931 (2010).
Paz-Filho, G., Mastronardi, C. A. & Licinio, J. Leptin treatment: facts and expectations. Metabolism. 64, 146–156 (2015).
Tchang, B. G., Shukla, A. P. & Aronne, L. J. Metreleptin and generalized lipodystrophy and evolving therapeutic perspectives. Expert Opin. Biol. Ther. 15, 1061–1075 (2015).
Matarese, G. et al. Selective capacity of metreleptin administration to reconstitute CD4+ T-cell number in females with acquired hypoleptinemia. Proc. Natl Acad. Sci. USA 110, E818–E827 (2013). This is a relevant article demonstrating important pharmacological properties of leptin in modulating immune response
Acknowledgements
O.G. and F.L. are Staff Personnel of Xunta de Galicia (SERGAS) through a research-staff stabilization contract (ISCIII/SERGAS). R.G. is a 'Miguel Servet' Researcher funded by Instituto de Salud Carlos III (ISCIII). O.G. is a member of RETICS (Redes Temáticas de Investigación Cooperativa en Salud) Programme, RD16/0012/0014, RIER (Red de Investigación en Inflamación y Enfermedades Reumáticas) via Instituto de Salud Carlos III and FEDER (Fondo Europeo de Desarrollo Regional). The work of O.G. (PIE 13/00024, PI14/00016), F.L. and R.G. is funded by Instituto de Salud Carlos III and FEDER. J.C. is a recipient of Sara Borrell Program funding by Instituto de Salud Carlos III (ISCIII). F.L. is a member of CIBERCV (Centro de Investigación Biomédica en Red de Enfermedades Cardio¬vasculares), funded by ISCIII and FEDER. The funders had no role in study design, data collection and analy¬sis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
O.G., V.A., M.S., J.C., M-A.G-G., J-J.G-R., A.M., and F.L. researched data for the article. J.P., M.-A.G.-G.,J.-J.G.-R., A.M., F.L., R.G contributed substantially to the discussion of the content, and O.G. and V.A. wrote the article. M.S., J.P., F.L., R.G., and O.G. reviewed and edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Anorexigenic factors
-
Mediators that reduce food intake by acting on hypothalamic receptors
- Orexigenic factors
-
Mediators that induce appetite and stimulate food intake
- Type 1 T helper cells
-
(TH1 cells). Subset of helper-inducer T-lymphocytes that synthesize and secrete IL-2, IFNγ and IL-12. Owing to their ability to kill antigen-presenting cells, TH1 cells are associated with vigorous delayed-type hypersensitivity reactions
- TH2 cells
-
Subset of helper-inducer T-lymphocytes that synthesize and secrete IL-4, IL-5, IL-6 and IL-10. These cytokines influence B-cell development and antibody production as well as augmenting humoral responses.
- Superoxides
-
Highly reactive compounds produced when oxygen is reduced by a single electron.
- Toll-like receptors
-
A family of pattern-recognition receptors characterized by an extracellular leucine-rich domain and a cytoplasmic domain that share homology with the IL-1 receptor and the drosophila toll protein. Following pathogen recognition, Toll-like receptors recruit and activate a variety of signal-transducing adaptor proteins.
- Hyperleptinaemia
-
Presence of a higher than normal amount of leptin in the bloodstream.
- Lymphopenia
-
Reduction in the number of lymphocytes
- Lipodystrophy
-
A collection of heterogenous conditions resulting from defective lipid metabolism and characterized by adipose tissue atrophy
- Hypothalamic amenorrhea
-
Hypogonadotropic hypogonadism related to an aberration of the pulsatile release of gonadotropin-releasing hormone from the hypothalamus
Rights and permissions
About this article
Cite this article
Abella, V., Scotece, M., Conde, J. et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol 13, 100–109 (2017). https://doi.org/10.1038/nrrheum.2016.209
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.209
This article is cited by
-
Macrophage and T cell networks in adipose tissue
Nature Reviews Endocrinology (2024)
-
Exploring the interplay of MTHFR and FGG polymorphisms with serum levels of adiponectin and leptin in pediatric lupus nephritis: a pilot study
Egyptian Journal of Medical Human Genetics (2024)
-
Leptin/obR signaling exacerbates obesity-related neutrophilic airway inflammation through inflammatory M1 macrophages
Molecular Medicine (2023)
-
Risk of metabolic abnormalities in osteoarthritis: a new perspective to understand its pathological mechanisms
Bone Research (2023)
-
Dynamic lipidome alterations associated with human health, disease and ageing
Nature Metabolism (2023)