Key Points
-
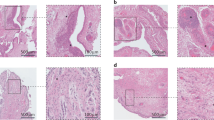
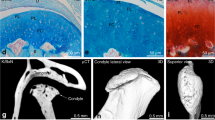
The molecular mechanisms of cartilage breakdown in rheumatoid arthritis (RA) and osteoarthritis (OA) show considerable overlap, particularly with respect to matrix-degrading enzymes, but also with respect to some inflammatory mediators
-
The loss of phenotypic stability of articular chondrocytes, and initiation of a programme resembling aspects of embryonic endochondral ossification, could explain important features of OA
-
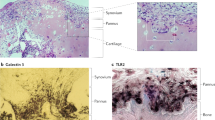
In contrast to OA, RA is associated with a stable, tumour-like activation of fibroblast-like synoviocytes that mediate the destruction of articular cartilage through directed invasion
-
Cartilage has active roles in OA and RA as a signalling scaffold harbouring bioactive matrix components and soluble factors, which interact with embedded chondrocytes and are released upon cartilage degradation
Abstract
Cartilage damage is a key feature of degenerative joint disorders—primarily osteoarthritis (OA)—and chronic inflammatory joint diseases, such as rheumatoid arthritis (RA). Substantial progress has been made towards understanding the mechanisms that lead to degradation of the cartilage matrix in either condition, which ultimately results in the progressive remodelling of affected joints. The available data have shown that the molecular steps in cartilage matrix breakdown overlap in OA and RA. However, they have also, to a great extent, changed our view of the roles of cartilage in the pathogenesis of these disorders. In OA, cartilage loss occurs as part of a complex programme that resembles aspects of embryonic bone formation through endochondral ossification. In RA, early cartilage damage is a key trigger of cellular reactions in the synovium. In a proposed model of RA as a site-specific manifestation of a systemic autoimmune disorder, early cartilage damage in the context of immune activation leads to a specific cellular response within articular joints that could explain not only the organ specificity of RA, but also the chronic nature and perpetuation of the disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pap, T., Korb-Pap, A., Heitzmann, M. & Bertrand, J. in Oxford Textbook of Rheumatology 4th edn Ch. 56 (eds Watts, R. A. et al.) pp. 409–414 (Oxford University Press, 2013).
Koziel, L., Kunath, M., Kelly, O. G. & Vortkamp, A. Ext1-dependent heparan sulfate regulates the range of Ihh signaling during endochondral ossification. Dev. Cell 6, 801–813 (2004).
Chuang, C. Y. et al. Heparan sulfate-dependent signaling of fibroblast growth factor 18 by chondrocyte-derived perlecan. Biochemistry 49, 5524–5532 (2010).
Sherwood, J. et al. A homeostatic function of CXCR2 signalling in articular cartilage. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2014-205546.
O'Conor, C. J., Leddy, H. A., Benefield, H. C., Liedtke, W. B. & Guilak, F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl Acad. Sci. USA 111, 1316–1321 (2014).
DeLise, A. M., Fischer, L. & Tuan, R. S. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage 8, 309–334 (2000).
Kerkhof, H. J. et al. Recommendations for standardization and phenotype definitions in genetic studies of osteoarthritis: the TREAT-OA consortium. Osteoarthritis Cartilage 19, 254–264 (2011).
Goldring, M. B. & Goldring, S. R. Osteoarthritis. J. Cell. Physiol. 213, 626–634 (2007).
Conaghan, P. G. Osteoarthritis in 2012: parallel evolution of OA phenotypes and therapies. Nat. Rev. Rheumatol. 9, 68–70 (2013).
Wang, M. et al. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann. NY Acad. Sci. 1240, 61–69 (2011).
Felson, D. T. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage 21, 10–15 (2013).
Wluka, A. E., Lombard, C. B. & Cicuttini, F. M. Tackling obesity in knee osteoarthritis. Nat. Rev. Rheumatol. 9, 225–235 (2013).
Meulenbelt, I., Kraus, V. B., Sandell, L. J. & Loughlin, J. Summary of the OA biomarkers workshop 2010 - genetics and genomics: new targets in OA. Osteoarthritis Cartilage 19, 1091–1094 (2011).
Zhai, G. et al. A genome-wide association study suggests that a locus within the ataxin 2 binding protein 1 gene is associated with hand osteoarthritis: the Treat-OA consortium. J. Med. Genet. 46, 614–616 (2009).
Troeberg, L. & Nagase, H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim. Biophys. Acta 1824, 133–145 (2012).
Smith, G. N. Jr. The role of collagenolytic matrix metalloproteinases in the loss of articular cartilage in osteoarthritis. Front. Biosci. 11, 3081–3095 (2006).
Verma, P. & Dalal, K. ADAMTS-4 and ADAMTS-5: key enzymes in osteoarthritis. J. Cell. Biochem. 112, 3507–3514 (2011).
De Croos, J. N. et al. Membrane type-1 matrix metalloproteinase is induced following cyclic compression of in vitro grown bovine chondrocytes. Osteoarthritis Cartilage 15, 1301–1310 (2007).
Honsawek, S. et al. Association of MMP-3 (-1612 5A/6A) polymorphism with knee osteoarthritis in Thai population. Rheumatol. Int. 33, 435–439 (2013).
Lin, P. M., Chen, C. T. & Torzilli, P. A. Increased stromelysin-1 (MMP-3), proteoglycan degradation (3B3- and 7D4) and collagen damage in cyclically load-injured articular cartilage. Osteoarthritis Cartilage 12, 485–496 (2004).
Huebner, J. L., Otterness, I. G., Freund, E. M., Caterson, B. & Kraus, V. B. Collagenase 1 and collagenase 3 expression in a guinea pig model of osteoarthritis. Arthritis Rheum. 41, 877–890 (1998).
Yoshihara, Y. et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann. Rheum. Dis. 59, 455–461 (2000).
Lim, N. H., Meinjohanns, E., Meldal, M., Bou-Gharios, G. & Nagase, H. In vivo imaging of MMP-13 activity in the murine destabilised medial meniscus surgical model of osteoarthritis. Osteoarthritis Cartilage 22, 862–868 (2014).
Tetlow, L. C., Adlam, D. J. & Woolley, D. E. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 44, 585–594 (2001).
Hirata, M. et al. C/EBPβ and RUNX2 cooperate to degrade cartilage with MMP-13 as the target and HIF-2α as the inducer in chondrocytes. Hum. Mol. Genet. 21, 1111–1123 (2012).
Burrage, P. S., Mix, K. S. & Brinckerhoff, C. E. Matrix metalloproteinases: role in arthritis. Front. Biosci. 11, 529–543 (2006).
Borden, P. et al. Cytokine control of interstitial collagenase and collagenase-3 gene expression in human chondrocytes. J. Biol. Chem. 271, 23577–23581 (1996).
Fan, Z., Yang, H., Bau, B., Söder, S. & Aigner, T. Role of mitogen-activated protein kinases and NFκB on IL-1β-induced effects on collagen type II, MMP-1 and 13 mRNA expression in normal articular human chondrocytes. Rheumatol. Int. 26, 900–903 (2006).
Neuhold, L. A. et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J. Clin. Invest. 107, 35–44 (2001).
Little, C. B. et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 60, 3723–3733 (2009).
Stanton, H. et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 434, 648–652 (2005).
Song, R. H. et al. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 56, 575–85 (2007).
Majumdar, M. K. et al. Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheum. 56, 3670–3674 (2007).
Yamanishi, Y. et al. Expression and regulation of aggrecanase in arthritis: the role of TGF-β. J. Immunol. 168, 1405–1412 (2002).
Wylie, J. D., Ho, J. C., Singh, S., McCulloch, D. R. & Apte, S. S. Adamts5 (aggrecanase-2) is widely expressed in the mouse musculoskeletal system and is induced in specific regions of knee joint explants by inflammatory cytokines. J. Orthop. Res. 30, 226–233 (2012).
Echtermeyer, F. et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat. Med. 15, 1072–1076 (2009).
Wang, J. et al. TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J. Biol. Chem. 286, 39738–39749 (2011).
Brew, C. J., Clegg, P. D., Boot-Handford, R. P., Andrew, J. G. & Hardingham, T. Gene expression in human chondrocytes in late osteoarthritis is changed in both fibrillated and intact cartilage without evidence of generalised chondrocyte hypertrophy. Ann. Rheum. Dis. 69, 234–240 (2010).
Dy, P. et al. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev. Cell 22, 597–609 (2012).
Leung, V. Y. et al. SOX9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression. PLoS Genet. 7, e1002356 (2011).
Yagi, R., McBurney, D., Laverty, D., Weiner, S. & Horton, W. E. Jr. Intrajoint comparisons of gene expression patterns in human osteoarthritis suggest a change in chondrocyte phenotype. J. Orthop. Res. 23, 1128–1138 (2005).
Haag, J., Gebhard, P. M. & Aigner, T. SOX gene expression in human osteoarthritic cartilage. Pathobiology 75, 195–199 (2008).
Salminen, H., Vuorio, E. & Säämänen, A. M. Expression of Sox9 and type IIA procollagen during attempted repair of articular cartilage damage in a transgenic mouse model of osteoarthritis. Arthritis Rheum. 44, 947–955 (2001).
Henry, S. P., Liang, S., Akdemir, K. C. & de Crombrugghe, B. The postnatal role of Sox9 in cartilage. J. Bone Miner. Res. 27, 2511–2525 (2012).
Tew, S. R. et al. Retroviral transduction with SOX9 enhances re-expression of the chondrocyte phenotype in passaged osteoarthritic human articular chondrocytes. Osteoarthritis Cartilage 13, 80–89 (2005).
Cucchiarini, M. et al. Restoration of the extracellular matrix in human osteoarthritic articular cartilage by overexpression of the transcription factor SOX9. Arthritis Rheum. 56, 158–167 (2007).
Pullig, O., Weseloh, G., Klatt, A. R., Wagener, R. & Swoboda, B. Matrilin-3 in human articular cartilage: increased expression in osteoarthritis. Osteoarthritis Cartilage 10, 253–263 (2002).
Stefánsson, S. E. et al. Genomewide scan for hand osteoarthritis: a novel mutation in matrilin-3. Am. J. Hum. Genet. 72, 1448–1459 (2003).
Eliasson, G. J., Verbruggen, G., Stefansson, S. E., Ingvarsson, T. & Jonsson, H. Hand radiology characteristics of patients carrying the T(303)M mutation in the gene for matrilin-3. Scand. J. Rheumatol. 35, 138–142 (2006).
Vincourt, J. B. et al. Increased expression of matrilin-3 not only in osteoarthritic articular cartilage but also in cartilage-forming tumors, and down-regulation of SOX9 via epidermal growth factor domain 1-dependent signaling. Arthritis Rheum. 58, 2798–2808 (2008).
Chintala, S. K., Miller, R. R. & McDevitt, C. A. Basic fibroblast growth factor binds to heparan sulfate in the extracellular matrix of rat growth plate chondrocytes. Arch. Biochem. Biophys. 310, 180–186 (1994).
Vincent, T. L., McLean, C. J., Full, L. E., Peston, D. & Saklatvala, J. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthritis Cartilage 15, 752–763 (2007).
Chia, S. L. et al. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 60, 2019–2027 (2009).
Kawaguchi, H. Endochondral ossification signals in cartilage degradation during osteoarthritis progression in experimental mouse models. Mol. Cells 25, 1–6 (2008).
Dreier, R. Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Res. Ther. 12, 216 (2010).
Karlsson, C., Brantsing, C., Egell, S. & Lindahl, A. Notch1, Jagged1, and HES5 are abundantly expressed in osteoarthritis. Cells Tissues Organs 188, 287–298 (2008).
Hosaka, Y. et al. Notch signaling in chondrocytes modulates endochondral ossification and osteoarthritis development. Proc. Natl Acad. Sci. USA 110, 1875–1880 (2013).
Lingaraj, K., Poh, C. K. & Wang, W. Vascular endothelial growth factor (VEGF) is expressed during articular cartilage growth and re-expressed in osteoarthritis. Ann. Acad. Med. Singapore 39, 399–403 (2010).
Mitchell, P. G. et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J. Clin. Invest. 97, 761–768 (1996).
Mirando, A. J. et al. RBP-Jκ-dependent Notch signaling is required for murine articular cartilage and joint maintenance. Arthritis Rheum. 65, 2623–2633 (2013).
Bertrand, J. et al. Syndecan 4 supports bone fracture repair, but not fetal skeletal development, in mice. Arthritis Rheum. 65, 743–752 (2013).
Ko, Y. et al. Matrilin-3 is dispensable for mouse skeletal growth and development. Mol. Cell Biol. 24, 1691–1699 (2004).
Saito, T. et al. Transcriptional regulation of endochondral ossification by HIF-2α during skeletal growth and osteoarthritis development. Nat. Med. 16, 678–686 (2010).
Araldi, E., Khatri, R., Giaccia, A. J., Simon, M. C. & Schipani, E. Lack of HIF-2α in limb bud mesenchyme causes a modest and transient delay of endochondral bone development. Nat. Med. 17, 25–26 (2011).
Nakajima, M. et al. Replication studies in various ethnic populations do not support the association of the HIF-2α SNP rs17039192 with knee osteoarthritis. Nat. Med. 17, 26–27 (2011).
Kamekura, S. et al. Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum. 54, 2462–2470 (2006).
Lin, A. C. et al. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat. Med. 15, 1421–1425 (2009).
Goldring, M. B. & Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 23, 471–478 (2011).
Wang, Q. et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 17, 1674–1679 (2011).
Edd, S. N., Giori, N. J. & Andriacchi, T. P. The role of inflammation in the initiation of osteoarthritis after meniscal damage. J. Biomech. 48, 1420–1426 (2015).
Gierman, L. M. et al. Metabolic stress-induced inflammation plays a major role in the development of osteoarthritis in mice. Arthritis Rheum. 64, 1172–1181 (2012).
Griffin, T. M., Huebner, J. L., Kraus, V. B., Yan, Z. & Guilak, F. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Arthritis Rheum. 64, 443–453 (2012).
Ashraf, S., Mapp, P. I. & Walsh, D. A. Contributions of angiogenesis to inflammation, joint damage, and pain in a rat model of osteoarthritis. Arthritis Rheum. 63, 2700–2710 (2011).
Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage 21, 16–21 (2013).
Bougault, C. et al. Stress-induced cartilage degradation does not depend on the NLRP3 inflammasome in human osteoarthritis and mouse models. Arthritis Rheum. 64, 3972–3981 (2012).
de Hooge, A. S. et al. Male IL-6 gene knock out mice developed more advanced osteoarthritis upon aging. Osteoarthritis Cartilage 13, 66–73 (2005).
Kim, H. A. et al. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 54, 2152–2163 (2006).
Liu-Bryan, R. & Terkeltaub, R. Chondrocyte innate immune myeloid differentiation factor 88-dependent signaling drives procatabolic effects of the endogenous Toll-like receptor 2/Toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein 1 in mice. Arthritis Rheum. 62, 2004–2012 (2010).
Gondokaryono, S. P. et al. The extra domain A of fibronectin stimulates murine mast cells via toll-like receptor 4. J. Leukoc. Biol. 82, 657–665 (2007).
Lefebvre, J. S. et al. Extra domain A of fibronectin primes leukotriene biosynthesis and stimulates neutrophil migration through activation of Toll-like receptor 4. Arthritis Rheum. 63, 1527–1533 (2011).
Midwood, K. et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 15, 774–780 (2009).
Schaefer, L. et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J. Clin. Invest. 115, 2223–2233 (2005).
Rushton, M. D. et al. Characterization of the cartilage DNA methylome in knee and hip osteoarthritis. Arthritis Rheumatol. 66, 2450–2460 (2014).
Moazedi-Fuerst, F. C. et al. Epigenetic differences in human cartilage between mild and severe OA. J. Orthop. Res. 32, 1636–1645 (2014).
Kim, K. I., Park, Y. S. & Im, G. I. Changes in the epigenetic status of the SOX-9 promoter in human osteoarthritic cartilage. J. Bone Miner. Res. 28, 1050–1060 (2013).
Imagawa, K. et al. Association of reduced type IX collagen gene expression in human osteoarthritic chondrocytes with epigenetic silencing by DNA hypermethylation. Arthritis Rheumatol. 66, 3040–3051 (2014).
Muller-Ladner, U., Pap, T., Gay, R. E., Neidhart, M. & Gay, S. Mechanisms of disease: the molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat. Clin. Pract. Rheumatol. 1, 102–110 (2005).
Rommel, C., Camps, M. & Ji, H. PI3Kδ and PI3Kγ: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat. Rev. Immunol. 7, 191–201 (2007).
Brennan, F. M. & McInnes, I. B. Evidence that cytokines play a role in rheumatoid arthritis. J. Clin. Invest. 118, 3537–3545 (2008).
Klareskog, L., Padyukov, L., Lorentzen, J. & Alfredsson, L. Mechanisms of disease: genetic susceptibility and environmental triggers in the development of rheumatoid arthritis. Nat. Clin. Pract. Rheumatol. 2, 425–433 (2006).
Kobezda, T., Ghassemi-Nejad, S., Mikecz, K., Glant, T. T. & Szekanecz, Z. Of mice and men: how animal models advance our understanding of T-cell function in RA. Nat. Rev. Rheumatol. 10, 160–170 (2014).
Huber, L. C. et al. Synovial fibroblasts: key players in rheumatoid arthritis. Rheumatology (Oxford) 45, 669–675 (2006).
Niedermeier, M., Pap, T. & Korb, A. Therapeutic opportunities in fibroblasts in inflammatory arthritis. Best Pract. Res. Clin. Rheumatol. 24, 527–540 (2010).
Bottini, N. & Firestein, G. S. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat. Rev. Rheumatol. 9, 24–33 (2013).
Pap, T. et al. Expression of membrane-type matrix metallo proteinases (MT-MMP), MMP-2 and MMP-13 in the rheumatoid arthritis (RA) synovium [abstract 106]. Arthritis Rheum. 42, S88 (1999).
Rutkauskaite, E. et al. Retroviral gene transfer of an antisense construct against membrane type 1 matrix metalloproteinase reduces the invasiveness of rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 52, 2010–2014 (2005).
Miller, M. C. et al. Membrane type 1 matrix metalloproteinase is a crucial promoter of synovial invasion in human rheumatoid arthritis. Arthritis Rheum. 60, 686–697 (2009).
Pap, T., Müller-Ladner, U., Gay, R. E. & Gay, S. Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2, 361–367 (2000).
Rinaldi, N. et al. Increased expression of integrins on fibroblast-like synoviocytes from rheumatoid arthritis in vitro correlates with enhanced binding to extracellular matrix proteins. Ann. Rheum. Dis. 56, 45–51 (1997).
Lowin, T. & Straub, R. H. Integrins and their ligands in rheumatoid arthritis. Arthritis Res. Ther. 13, 244 (2011).
Patterson, A. M. et al. Differential expression of syndecans and glypicans in chronically inflamed synovium. Ann. Rheum. Dis. 67, 592–601 (2008).
Kehoe, O. et al. Syndecan-3 is selectively pro-inflammatory in the joint and contributes to antigen-induced arthritis in mice. Arthritis Res. Ther. 16, R148 (2014).
Korb-Pap, A. et al. Early structural changes in cartilage and bone are required for the attachment and invasion of inflamed synovial tissue during destructive inflammatory arthritis. Ann. Rheum. Dis. 71, 1004–1011 (2012).
Peters, M. A. et al. The loss of α2β1 integrin suppresses joint inflammation and cartilage destruction in mouse models of rheumatoid arthritis. Arthritis Rheum. 64, 1359–1368 (2012).
Zwerina, J. et al. TNF-induced structural joint damage is mediated by IL-1. Proc. Natl Acad. Sci. USA 104, 11742–11747 (2007).
Shiozawa, S. et al. Pathogenic importance of fibronectin in the superficial region of articular cartilage as a local factor for the induction of pannus extension on rheumatoid articular cartilage. Ann. Rheum. Dis. 51, 869–873 (1992).
Yasuda, T. Cartilage destruction by matrix degradation products. Mod. Rheumatol. 16, 197–205 (2006).
Shelef, M. A., Bennin, D. A., Mosher, D. F. & Huttenlocher, A. Citrullination of fibronectin modulates synovial fibroblast behavior. Arthritis Res. Ther. 14, R240 (2012).
Sipilä, K. et al. Citrullination of collagen II affects integrin-mediated cell adhesion in a receptor-specific manner. FASEB J. 28, 3758–3768 (2014).
Podolin, P. L. et al. A potent and selective nonpeptide antagonist of CXCR2 inhibits acute and chronic models of arthritis in the rabbit. J. Immunol. 169, 6435–6444 (2002).
Grespan, R. et al. CXCR2-specific chemokines mediate leukotriene B4-dependent recruitment of neutrophils to inflamed joints in mice with antigen-induced arthritis. Arthritis Rheum. 58, 2030–2040 (2008).
Manabe, N. et al. Involvement of fibroblast growth factor-2 in joint destruction of rheumatoid arthritis patients. Rheumatology (Oxford) 38, 714–720 (1999).
Yamashita, A. et al. Fibroblast growth factor-2 determines severity of joint disease in adjuvant-induced arthritis in rats. J. Immunol. 168, 450–457 (2002).
Nakano, K., Okada, Y., Saito, K. & Tanaka, Y. Induction of RANKL expression and osteoclast maturation by the binding of fibroblast growth factor 2 to heparan sulfate proteoglycan on rheumatoid synovial fibroblasts. Arthritis Rheum. 50, 2450–2458 (2004).
Abe, K., Aslam, A., Walls, A. F., Sato, T. & Inoue, H. Up-regulation of protease-activated receptor-2 by bFGF in cultured human synovial fibroblasts. Life Sci. 79, 898–904 (2006).
Raza, K. et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res. Ther. 7, R784–R795 (2005).
Pap, T. & Gay, S. in Kelley's Textbook of Rheumatology 8th edn (eds Firestein, G. S. et al.) 201–214 (Saunders Elsevier, 2009).
Karouzakis, E., Gay, R. E., Michel, B. A., Gay, S. & Neidhart, M. DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 60, 3613–3622 (2009).
Nakano, K., Whitaker, J. W., Boyle, D. L., Wang, W. & Firestein, G. S. DNA methylome signature in rheumatoid arthritis. Ann. Rheum. Dis. 72, 110–117 (2013).
Wada, T. T. et al. Aberrant histone acetylation contributes to elevated interleukin-6 production in rheumatoid arthritis synovial fibroblasts. Biochem. Biophys. Res. Commun. 444, 682–686 (2014).
Karouzakis, E. et al. Epigenome analysis reveals TBX5 as a novel transcription factor involved in the activation of rheumatoid arthritis synovial fibroblasts. J. Immunol. 193, 4945–4951 (2014).
Frank, S. et al. Regulation of matrixmetalloproteinase-3 and matrixmetalloproteinase-13 by SUMO-2/3 through the transcription factor NF-κB. Ann. Rheum. Dis. 72, 1874–1881 (2013).
Li, F. et al. SUMO-conjugating enzyme UBC9 promotes proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis. Inflammation 37, 1134–1141 (2014).
Maciejewska-Rodrigues, H. et al. Epigenetics and rheumatoid arthritis: the role of SENP1 in the regulation of MMP-1 expression. J. Autoimmun. 35, 15–22 (2010).
Meinecke, I. et al. Modification of nuclear PML protein by SUMO-1 regulates Fas-induced apoptosis in rheumatoid arthritis synovial fibroblasts. Proc. Natl Acad. Sci. USA 104, 5073–5078 (2007).
Kato, M., Ospelt, C., Gay, R. E., Gay, S. & Klein, K. Dual role of autophagy in stress-induced cell death in rheumatoid arthritis synovial fibroblasts. Arthritis Rheumatol. 66, 40–48 (2014).
Nakano, K., Boyle, D. L. & Firestein, G. S. Regulation of DNA methylation in rheumatoid arthritis synoviocytes. J. Immunol. 190, 1297–1303 (2013).
Angiolilli, C. et al. Inflammatory cytokines epigenetically regulate rheumatoid arthritis fibroblast-like synoviocyte activation by suppressing HDAC5 expression. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2014-205635.
Patel, R., Filer, A., Barone, F. & Buckley, C. D. Stroma: fertile soil for inflammation. Best Pract. Res. Clin. Rheumatol. 28, 565–576 (2014).
Lefèvre, S. et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat. Med. 15, 1414–1420 (2009).
Krzeski, P. et al. Development of musculoskeletal toxicity without clear benefit after administration of PG-116800, a matrix metalloproteinase inhibitor, to patients with knee osteoarthritis: a randomized, 12-month, double-blind, placebo-controlled study. Arthritis Res. Ther. 9, R109 (2007).
Doherty, M. & Dieppe, P. The “placebo” response in osteoarthritis and its implications for clinical practice. Osteoarthritis Cartilage 17, 1255–1262 (2009).
Abhishek, A. & Doherty, M. Mechanisms of the placebo response in pain in osteoarthritis. Osteoarthritis Cartilage 21, 1229–1235 (2013).
Wojtowicz-Praga, S. Clinical potential of matrix metalloprotease inhibitors. Drugs R. D. 1, 117–129 (1999).
Acknowledgements
T.P. received funding from the German Research Foundation (SFB1009 TP08 and PA689/7). T.P. and A.K.P. received funding from the German National Ministry of Education and Research (01EC1008D).
Author information
Authors and Affiliations
Contributions
Both authors researched the data for the article and provided a substantial contribution to discussions of the content, and contributed equally to writing the article and to review and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Pap, T., Korb-Pap, A. Cartilage damage in osteoarthritis and rheumatoid arthritis—two unequal siblings. Nat Rev Rheumatol 11, 606–615 (2015). https://doi.org/10.1038/nrrheum.2015.95
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2015.95
This article is cited by
-
Inhibitory effects of Acanthopanax sessiliflorus Harms extract on the etiology of rheumatoid arthritis in a collagen-induced arthritis mouse model
Arthritis Research & Therapy (2024)
-
Nerve growth factor receptor limits inflammation to promote remodeling and repair of osteoarthritic joints
Nature Communications (2024)
-
Targeting macrophagic PIM-1 alleviates osteoarthritis by inhibiting NLRP3 inflammasome activation via suppressing mitochondrial ROS/Cl− efflux signaling pathway
Journal of Translational Medicine (2023)
-
Imaging in inflammatory arthritis: progress towards precision medicine
Nature Reviews Rheumatology (2023)
-
Inhibition of fibroblast activation protein ameliorates cartilage matrix degradation and osteoarthritis progression
Bone Research (2023)