Key Points
-
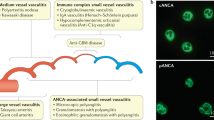
Screening for antineutrophil cytoplasmic antibodies (ANCAs) specific for proteinase 3 (PR3) and myeloperoxidase (MPO) remains a basic tool in the serological evaluation of systemic vasculitic disorders
-
Automated analysis of ANCA immunofluorescence demonstrates good agreement with conventional techniques when assessing patients with suspected vasculitis and, in the future, might improve efficiency and standardization in clinical laboratories
-
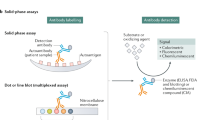
Antigen-specific ANCA assays that use bridging molecules exhibit superior performance compared with conventional assays and can be used for PR3-ANCA and MPO-ANCA detection
-
The new methods for ANCA detection and evaluation in ANCA-associated vasculitis should be urgently evaluated in multicentre studies, in anticipation of updating of standardization processes and a revision of existing strategies
Abstract
Detection of antineutrophil cytoplasmic antibodies (ANCAs) is a well-established diagnostic test used to evaluate suspected necrotizing vasculitis of small blood vessels. Conditions associated with these antibodies, collectively referred to as ANCA-associated vasculitides, include granulomatosis with polyangiitis (formerly known as Wegener granulomatosis), microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis (formerly known as Churg–Strauss syndrome). The diagnostic utility of ANCA testing depends on the type of assay performed and on the clinical setting. Most laboratories worldwide use standard indirect immunofluorescence tests (IFT) to screen for ANCA and then confirm positive IFT results with antigen-specific tests for proteinase 3 (PR3) and myeloperoxidase (MPO). Developments such as automated image analysis of immunofluorescence patterns, so-called third-generation PR3-ANCA and MPO-ANCA ELISA, and multiplex technology have improved the detection of ANCAs. However, challenges in routine clinical practice remain, including methodological aspects of IFT performance, the diverse antigen-specific assays available, the diagnostic value of testing in clinical settings and the prognostic value of serial ANCA monitoring in the prediction of disease relapse. This Review summarizes the available data on ANCA testing, discusses the usefulness of the various ANCA assays and advises on the clinical indications for the use of ANCA testing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Davies, D. J. et al. Segmental necrotizing glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? Br. Med. J. (Clin. Res. Ed.) 285, 606 (1982).
Van der Woude, F. J., Rasmussen, N. & Lobatto, S. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet 1, 425–429 (1985).
Jennette, J. C. et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 37, 187–192 (1994).
Jennette, J. C. et al. 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 65, 1–11 (2013).
Schmitt, W. H. & van der Woude, F. J. Clinical applications of antineutrophil cytoplasmic antibody testing. Curr. Opin. Rheumatol. 16, 9–17 (2004).
Csernok, E., Lamprecht, P. & Gross, W. L. Diagnostic significance of ANCA in vasculitis. Nat. Clin. Pract. Rheumatol. 2, 174–175 (2006).
Cohen Tervaert, J. W. & Damoiseaux, J. Antineutrophil cytoplasmic autoantibodies: how are they detected and what is their use for diagnosis, classification and follow-up? Clin. Rev. Allergy Immunol. 43, 211–219 (2012).
Specks, U. Controversies in ANCA testing. Cleve. Clin. J. Med. 79 (Suppl. 3), S7–S11 (2012).
Savige, J. et al. International Consensus Statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA). Am. J. Clin. Pathol. 111, 507–513 (1999).
Savige, J. et al. Addendum to the International Consensus Statement on testing and reporting of anti-neutrophil cytoplasmatic antibodies. Quality control guidelines, comments and recommendations for testing in other autoimmune diseases. Am. J. Clin. Pathol. 120, 312–314 (2003).
Hagen, E. C. et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int. 53, 743–753 (1998).
Csernok, E. & Holle, J. U. Twenty-eight years with antineutrophil cytoplasmic antibodies (ANCA): how to test for ANCA—evidence-based immunology? Autoimmunity Highlights 1, 39–43 (2010).
Russell, K. A., Wiegert, E., Schroeder, D. R., Homburger, H. A. & Specks, U. Detection of anti-neutrophil cytoplasmic antibodies under actual clinical testing conditions. Clin. Immunol. 103, 196–203 (2002).
Willitzki, A. et al. New platform technology for comprehensive serological diagnostics of autoimmune diseases. Clin. Dev. Immunol. http://dx.doi.org/10.1155/2012/284740 (2012).
Boomsma, M. M. et al. Image analysis: a novel approach for the quantification of ANCA levels in patients with Wegener's granulomatosis. J. Immunol. Methods 274, 27–35 (2003).
Melegari, A. et al. A comparative study on the reliability of an automated system for the evaluation of cell-based indirect immunofluorescence. Autoimmun. Rev. 11, 713–716 (2012).
Knütter, I. et al. Automated interpretation of ANCA patterns—a new approach in the serology of ANCA-associated vasculitis. Arthritis Res Ther. 14, R271 (2012).
Damoiseaux, J., Mallet, K., Vaessen. M., Austen, J. & Cohen Tervaert, J. W. Automatic reading of ANCA-slides: evaluation of the AKLIDES system. Clin. Dev. Immunol. http://dx.doi.org/10.1155/2012/762874 (2012).
Franssen, C. et al. Disease spectrum of patients with antineutrophil cytoplasmic autoantibodies of defined specificity: distinct differences between patients with anti-proteinase 3 and anti-myeloperoxidase autoantibodies. J. Intern. Med. 244, 209–216 (1998).
Schönermarck, U., Lamprecht, P., Csernok, E. & Gross, W. L. Prevalence and spectrum of rheumatic diseases associated with proteinase 3-antineutrophil cytoplasmic antibodies (ANCA) and myeloperoxidase-ANCA. Rheumatology (Oxford) 40, 178–184 (2001).
Hauer, H. A. et al. Renal histology in ANCA-associated vasculitis: differences between diagnostic and serologic subgroups. Kidney Int. 61, 80–89 (2002).
Lyons, P. A. et al. Genetically distinct subsets within ANCA-associated vasculitis. N. Engl. J. Med. 367, 214–223 (2012).
Holle, J. U., Hellmich, B., Backes, M., Gross, W. L. & Csernok, E. Variations in performance characteristics of commercial enzyme immunoassay kits of the detection of antineutrophil cytoplasmatic antibodies: what is the optimal cut-off? Ann. Rheum. Dis. 64. 1773–1779 (2005).
Westman, K. et al. Clinical evaluation of a capture-ELISA for PR3-ANCA. Kidney Int. 53, 1230–1236 (1998).
Sun, J. et al. Capture-ELISA based on recombinant PR3 is sensitive for PR3-ANCA testing and allows detection of PR3 and PR3-ANCA/PR3 immunocomplexes. J. Immunol. Meth. 211, 111–123 (1998).
Csernok, E. et al. Evaluation of capture ELISA for detection of antineutrophil cytoplasmic antibodies against proteinase-3 in Wegener's granulomatosis: first results from a multicenter study. Rheumatology (Oxford) 43, 174–180 (2004).
Gisslen, K., Wieslander, J., Westberg, K. & Herlitz, H. Relationship between anti-neutrophil cytoplasmatic antibody determined with conventional binding and the capture assay, and long-term clinical course in vasculitis. J. Int. Med. 251, 129–135 (2002).
Hellmich, B., Csernok, E., Fredenhagen, G. & Gross, W. L. A novel high sensitivity ELISA for detection of antineutrophil cytoplasm antibodies against proteinase-3. Clin. Exp. Rheumatol. 25 (1 Suppl. 44), S1–S5 (2007).
Holle, J. U., Csernok, E., Fredenhagen, G. & Gross, W. L. Clinical evaluation of hsPR3-ANCA ELISA for detection of antineutrophil cytoplasmic antibodies directed against proteinase 3. Ann. Rheum. Dis. 69, 468–469 (2010).
Damoiseaux, J. et al. A novel enzyme-linked immunosorbent assay using a mixture of human native and recombinant proteinase-3 significantly improves the diagnostic potential for antineutrophil cytoplasmic antibody-associated vasculitis. Ann. Rheum. Dis. 68, 228–233 (2009).
Roggenbuck, D. et al. High sensitivity detection of autoantibodies against proteinase 3 by a novel third-generation enzyme-linked immunoabsorbent assay. Ann. NY Acad Sci. 1173, 41–46 (2009).
Holle, J. U., Hermann K., Gross, W. L. & Csernok, E. Comparative analysis of different commercial ELISA systems for detection of anti-neutrophil cytoplasm antibodies in ANCA-associated vasculitides. Clin. Exp. Rheumatol. 30 (Suppl. 70), S66–S69 (2012).
Radice, A., Bianchi, L., Maggiore, U., Vaglio, A. & Sinico, R. A. Comparison of PR3-ANCA specific assay performance for the diagnosis of granulomatosis with polyangiitis (Wegener's). Clin. Chem. Lab. Med. 51, 2141–2149 (2013).
Vermeersch, P. et al. Determination of anti-neutrophil cytoplasmic antibodies in small vessel vasculitis: comparative analysis of different strategies. Clin. Chim. Acta. 397, 77–81 (2008).
Damoiseaux, J. et al. EUROPLUS ANCA BIOCHIP mosaic: PR3 and MPO antigen microdots improve the laboratory diagnostics of ANCA-associated vasculitis. J. Immunol. Meth. 348, 67–73 (2009).
Trevisin, M., Pollock, W. & Savige, J. Evaluation of a multiplex flow cytometric immunoassay to detect PR3- and MPO-ANCA in active and treated vasculitis, and in inflammatory bowel disease. J. Immunol. Meth. 336, 104–112 (2008).
Finkielman, J. D. et al. ANCA are detectable in nearly all patients with active severe Wegener's granulomatosis. Am. J. Med. 120, 643.e9–643.e14 (2007).
Westman, K. W. et al. Rapid screening assay for anti-GBM antibody and ANCAs; an important tool for the differential diagnosis of pulmonary renal syndromes. Nephrol. Dial. Transplant. 12, 1863–1868 (1997).
Tomasson, G. et al. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis: a meta-analysis. Rheumatology (Oxford) 51, 100–109 (2012).
McLaren, J. S., Stimson, R. H., McRorie, E. R., Coia, J. E. & Luqmani, R. A. The diagnostic value of anti-neutrophil cytoplasmic antibody testing in a routine clinical setting. QJM 94, 615–621 (2001).
Mandl, L. A. Using antineutrophil cytoplasmic antibody testing to diagnose vasculitis: can test-ordering guidelines improve diagnostic accuracy? Arch. Intern. Med. 162, 1509–1514 (2002).
Sinclair, D., Saas M. & Stevens, J. M. The effect of a symptom related ''gating policy'' on ANCA requests in routine clinical practice. J. Clin. Pathol. 57, 131–134 (2004).
Arnold, D. F. et al. Does a gating policy for ANCA overlook patients with ANCA associated vasculitis? An audit of 263 patients. J. Clin. Pathol. 63, 678–680 (2010).
Author information
Authors and Affiliations
Contributions
E.C. researched data for and wrote the article, F.M. made a substantial contribution to discussion of content, and both authors reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Csernok, E., Moosig, F. Current and emerging techniques for ANCA detection in vasculitis. Nat Rev Rheumatol 10, 494–501 (2014). https://doi.org/10.1038/nrrheum.2014.78
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2014.78
This article is cited by
-
Increased prevalence rate of metabolic syndrome is an independent predictor of cardiovascular disease in patients with antineutrophil cytoplasmic antibody-associated vasculitis
Rheumatology International (2022)
-
Unclassifiable repeated antineutrophil cytoplasmic antibody (ANCA) positivity in diseases other than ANCA-associated vasculitis
Zeitschrift für Rheumatologie (2022)
-
Levamisole and ANCA positivity in childhood nephrotic syndrome
Pediatric Nephrology (2021)
-
ANCA-Diagnostik bei Vaskulitiden
Zeitschrift für Rheumatologie (2020)
-
Evaluation of 12 different assays for detecting ANCA in Chinese patients with GPA and MPA: a multicenter study in China
Clinical Rheumatology (2019)