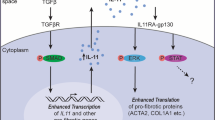
Abstract
Interleukin (IL)-33 is a member of the IL-1 family of cytokines. IL-33 is a nuclear protein that is also released into the extracellular space, and thus acts as a dual-function molecule, as does IL-1α. Extracellular IL-33 binds to the cell-surface receptor ST2, leading to the activation of intracellular signaling pathways similar to those used by IL-1. Unlike conventional cytokines, IL-33 might be secreted via unconventional pathways, and can be released upon cell injury as an alarmin. IL-33 is expressed in cells that are in contact with the environment, and acts as an early inducer of inflammation. Its production is then upregulated in inflamed tissues, thus contributing to the further amplification of inflammatory responses. Studies of IL-33-deficient mice will provide more information on intracellular functions of this cytokine. A large body of evidence supports the pathogenic role of IL-33 in asthma and possibly other inflammatory airway conditions. Furthermore, IL-33 has been shown to be involved in experimental models of arthritis and potentially has a pathogenic role in ulcerative colitis and fibrotic conditions, suggesting that IL-33 antagonists might be of interest for the treatment of asthma, rheumatoid arthritis and ulcerative colitis. However, IL-33 also appears to exert important functions in host defense against pathogens and to display cardioprotective properties, which might have implications for the clinical use of IL-33 blockade.
Key Points
-
Interleukin (IL)-33 is a member of the IL-1 family that is expressed as a 30 kD protein in the cell nucleus
-
IL-33 is not secreted following a conventional secretory pathway but is released as an alarmin following cell injury
-
Levels of IL-33 are elevated in the bronchial tissue of patients with asthma, and IL-33 is involved in experimental models of the disease, primarily by stimulating cells of the innate immune response
-
IL-33 levels are increased in rheumatoid arthritis, and IL-33 signaling is involved in the pathogenesis of several experimental models of arthritis
-
IL-33 contributes to host defense against parasitic and bacterial infections and exerts cardioprotective effects
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schmitz, J. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 (2005).
Moussion, C., Ortega, N. & Girard, J. P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PLoS ONE 3, e3331 (2008).
Carriere, V. et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl Acad. Sci. USA 104, 282–287 (2007).
Roussel, L., Erard, M., Cayrol, C. & Girard, J. P. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A–H2B acidic pocket. EMBO Rep. 9, 1006–1012 (2008).
Cayrol, C. & Girard, J. P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl Acad. Sci. USA 106, 9021–9026 (2009).
Talabot-Ayer, D., Lamacchia, C., Gabay, C. & Palmer, G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J. Biol. Chem. 284, 19420–19426 (2009).
Lüthi, A. U. et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 31, 84–98 (2009).
Oboki, K. et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc. Natl Acad. Sci. USA 107, 18581–18586 (2010).
Lingel, A. et al. Structure of IL-33 and its interaction with the ST2 and IL-1RAcP receptors—insight into heterotrimeric IL-1 signaling complexes. Structure 17, 1398–1410 (2009).
Baekkevold, E. S. et al. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am. J. Pathol. 163, 69–79 (2003).
Kuchler, A. M. et al. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am. J. Pathol. 173, 1229–1242 (2008).
Palmer, G. et al. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 60, 738–749 (2009).
Sponheim, J. et al. Inflammatory bowel disease-associated interleukin-33 is preferentially expressed in ulceration-associated myofibroblasts. Am. J. Pathol. 177, 2804–2815 (2010).
Ohno, T. et al. Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages. J. Immunol. 183, 7890–7897 (2009).
Hayakawa, M. et al. Mature interleukin-33 is produced by calpain-mediated cleavage in vivo. Biochem. Biophys. Res. Commun. 387, 218–222 (2009).
Ali, S., Nguyen, D. Q., Falk, W. & Martin, M. U. Caspase 3 inactivates biologically active full length interleukin-33 as a classical cytokine but does not prohibit nuclear translocation. Biochem. Biophys. Res. Commun. 391, 1512–1516 (2010).
Nile, C. J., Barksby, E., Jitprasertwong, P., Preshaw, P. M. & Taylor, J. J. Expression and regulation of interleukin-33 in human monocytes. Immunology 130, 172–180 (2010).
Matsuyama, Y. et al. Increased levels of interleukin 33 in sera and synovial fluid from patients with active rheumatoid arthritis. J. Rheumatol. 37, 18–25 (2010).
Haraldsen, G., Balogh, J., Pollheimer, J., Sponheim, J. & Kuchler, A. M. Interleukin-33—cytokine of dual function or novel alarmin? Trends Immunol. 30, 227–233 (2009).
Lamkanfi, M. & Dixit, V. M. IL-33 raises alarm. Immunity 31, 5–7 (2009).
Hudson, C. A. et al. Induction of IL-33 expression and activity in central nervous system glia. J. Leukoc. Biol. 84, 631–643 (2008).
Kobori, A. et al. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J. Gastroenterol. 45, 999–1007 (2010).
Kouzaki, H., Iijima, K., Kobayashi, T., O'Grady, S. M. & Kita, H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate TH2-type responses. J. Immunol. doi:10.4049/jimmunol.1003020.
Keller, M., Ruegg, A., Werner, S. & Beer, H. D. Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831 (2008).
Prefontaine, D. et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J. Immunol. 183, 5094–5103 (2009).
Pastorelli, L. et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental TH1/TH2 driven enteritis. Proc. Natl Acad. Sci. USA 107, 8017–8022 (2010).
Prefontaine, D. et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J. Allergy Clin. Immunol. 125, 752–754 (2010).
Wood, I. S., Wang, B. & Trayhurn, P. IL-33, a recently identified interleukin-1 gene family member, is expressed in human adipocytes. Biochem. Biophys. Res. Commun. 384, 105–109 (2009).
Reh, D. D., Wang, Y., Ramanathan, M. Jr. & Lane, A. P. Treatment-recalcitrant chronic rhinosinusitis with polyps is associated with altered epithelial cell expression of interleukin-33. Am. J. Rhinol. Allergy 24, 105–109 (2010).
Shimosato, T. et al. CpG oligodeoxynucleotides induce strong up-regulation of interleukin 33 via Toll-like receptor 9. Biochem. Biophys. Res. Commun. 394, 81–86 (2010).
Oboki, K., Ohno, T., Kajiwara, N., Saito, H. & Nakae, S. IL-33 and IL-33 receptors in host defense and diseases. Allergol. Int. 59, 143–160 (2010).
Trajkovic, V., Sweet, M. J. & Xu, D. T1/ST2—an IL-1 receptor-like modulator of immune responses. Cytokine Growth Factor Rev. 15, 87–95 (2004).
Bergers, G., Reikerstorfer, A., Braselmann, S., Graninger, P. & Busslinger, M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J. 13, 1176–1188 (1994).
Li, H. et al. The cloning and nucleotide sequence of human ST2L cDNA. Genomics 67, 284–290 (2000).
Hayakawa, H., Hayakawa, M., Kume, A. & Tominaga, S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J. Biol. Chem. 282, 26369–26380 (2007).
Yanagisawa, K., Tsukamoto, T., Takagi, T. & Tominaga, S. Murine ST2 gene is a member of the primary response gene family induced by growth factors. FEBS Lett. 302, 51–53 (1992).
Kumar, S., Tzimas, M. N., Griswold, D. E. & Young, P. R. Expression of ST2, an interleukin-1 receptor homologue, is induced by proinflammatory stimuli. Biochem. Biophys. Res. Commun. 235, 474–478 (1997).
Weinberg, E. O. et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation 106, 2961–2966 (2002).
Tago, K. et al. Tissue distribution and subcellular localization of a variant form of the human ST2 gene product, ST2V. Biochem. Biophys. Res. Commun. 285, 1377–1383 (2001).
Ali, S. et al. IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proc. Natl Acad. Sci. USA 104, 18660–18665 (2007).
Chackerian, A. A. et al. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J. Immunol. 179, 2551–2555 (2007).
Palmer, G. et al. The IL-1 receptor accessory protein (AcP) is required for IL-33 signaling and soluble AcP enhances the ability of soluble ST2 to inhibit IL-33. Cytokine 42, 358–364 (2008).
Ho, L. H. et al. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcεRI signals. J. Leukoc. Biol. 82, 1481–1490 (2007).
Pecaric-Petkovic, T., Didichenko, S. A., Kaempfer, S., Spiegl, N. & Dahinden, C. A. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood 113, 1526–1534 (2009).
Yagami, A. et al. IL-33 mediates inflammatory responses in human lung tissue cells. J. Immunol. 185, 5743–5750 (2010).
Chow, J. Y., Wong, C. K., Cheung, P. F. & Lam, C. W. Intracellular signaling mechanisms regulating the activation of human eosinophils by the novel TH2 cytokine IL-33: implications for allergic inflammation. Cell. Mol. Immunol. 7, 26–34 (2010).
Yndestad, A. et al. Modulation of interleukin signalling and gene expression in cardiac myocytes by endothelin-1. Int. J. Biochem. Cell Biol. 42, 263–272 (2010).
Tare, N. et al. KU812 cells provide a novel in vitro model of the human IL-33/ST2L axis: functional responses and identification of signaling pathways. Exp. Cell Res. 316, 2527–2537 (2010).
Sanada, S. et al. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Invest. 117, 1538–1549 (2007).
Funakoshi-Tago, M. et al. TRAF6 is a critical signal transducer in IL-33 signaling pathway. Cell. Signal. 20, 1679–1686 (2008).
Funakoshi-Tago, M., Tago, K., Sato, Y., Tominaga, S. I. & Kasahara, T. JAK2 is an important signal transducer in IL-33-induced NF-κB activation. Cell. Signal. 23, 363–370 (2010).
Bulek, K. et al. The essential role of single Ig IL-1 receptor-related molecule/Toll IL-1R8 in regulation of TH2 immune response. J. Immunol. 182, 2601–2609 (2009).
Drube, S. et al. The receptor tyrosine kinase c-Kit controls IL-33 receptor signaling in mast cells. Blood 115, 3899–3906 (2010).
Mun, S. H. et al. Interleukin-33 stimulates formation of functional osteoclasts from human CD14+ monocytes. Cell. Mol. Life Sci. 67, 3883–3892 (2010).
Takayanagi, H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 7, 292–304 (2007).
Ivashkiv, L. B. A signal-switch hypothesis for cross-regulation of cytokine and TLR signalling pathways. Nat. Rev. Immunol. 8, 816–822 (2008).
Smith, D. E. The biological paths of IL-1 family members IL-18 and IL-33. J. Leukoc. Biol. 89, 383–392 (2010).
Iikura, M. et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab. Invest. 87, 971–978 (2007).
Moulin, D. et al. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine 40, 216–225 (2007).
Komai-Koma, M. et al. IL-33 is a chemoattractant for human TH2 cells. Eur. J. Immunol. 37, 2779–2786 (2007).
Kurowska-Stolarska, M. et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J. Immunol. 181, 4780–4790 (2008).
Cherry, W. B., Yoon, J., Bartemes, K. R., Iijima, K. & Kita, H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J. Allergy Clin. Immunol. 121, 1484–1490 (2008).
Smithgall, M. D. et al. IL-33 amplifies both TH1- and TH2-type responses through its activity on human basophils, allergen-reactive TH2 cells, iNKT and NK cells. Int. Immunol. 20, 1019–1030 (2008).
Suzukawa, M. et al. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J. Immunol. 181, 5981–5989 (2008).
Guo, L. et al. IL-1 family members and STAT activators induce cytokine production by TH2, TH17, and TH1 cells. Proc. Natl Acad. Sci. USA 106, 13463–13468 (2009).
Kroeger, K. M., Sullivan, B. M. & Locksley, R. M. IL-18 and IL-33 elicit TH2 cytokines from basophils via a MyD88- and p38α-dependent pathway. J. Leukoc. Biol. 86, 769–778 (2009).
Schneider, E. et al. IL-33 activates unprimed murine basophils directly in vitro and induces their in vivo expansion indirectly by promoting hematopoietic growth factor production. J. Immunol. 183, 3591–3597 (2009).
Allakhverdi, Z. et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J. Allergy Clin. Immunol. 123, 472–478 (2009).
Rank, M. A. et al. IL-33-activated dendritic cells induce an atypical TH2-type response. J. Allergy Clin. Immunol. 123, 1047–1054 (2009).
Espinassous, Q. et al. IL-33 enhances lipopolysaccharide-induced inflammatory cytokine production from mouse macrophages by regulating lipopolysaccharide receptor complex. J. Immunol. 183, 1446–1455 (2009).
Kurowska-Stolarska, M. et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J. Immunol. 183, 6469–6477 (2009).
Bourgeois, E. et al. The pro-TH2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-γ production. Eur. J. Immunol. 39, 1046–1055 (2009).
Kim, W. et al. A novel method for procuring a large quantity of mature murine eosinophils in vivo. J. Immunol. Methods 363, 90–94 (2010).
Schulze, J. et al. Interleukin-33 is expressed in differentiated osteoblasts and blocks osteoclast formation from bone marrow precursor cells. J. Bone Miner. Res. doi:10.1002/jbmr.269.
Joshi, A. D. et al. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 11, 52 (2010).
Humphreys, N. E., Xu, D., Hepworth, M. R., Liew, F. Y. & Grencis, R. K. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J. Immunol. 180, 2443–2449 (2008).
Pushparaj, P. N. et al. The cytokine interleukin-33 mediates anaphylactic shock. Proc. Natl Acad. Sci. USA 106, 9773–9778 (2009).
Matsuba-Kitamura, S. et al. Contribution of IL-33 to induction and augmentation of experimental allergic conjunctivitis. Int. Immunol. 22, 479–489 (2010).
Yin, H. et al. IL-33 prolongs murine cardiac allograft survival through induction of TH2-type immune deviation. Transplantation 89, 1189–1197 (2010).
Rankin, A. L. et al. IL-33 induces IL-13-dependent cutaneous fibrosis. J. Immunol. 184, 1526–1535 (2010).
Hazlett, L. D. et al. IL-33 shifts macrophage polarization, promoting resistance against Pseudomonas aeruginosa keratitis. Invest. Ophthalmol. Vis. Sci. 51, 1524–1532 (2010).
Stolarski, B., Kurowska-Stolarska, M., Kewin, P., Xu, D. & Liew, F. Y. IL-33 exacerbates eosinophil-mediated airway inflammation. J. Immunol. 185, 3472–3480 (2010).
Kondo, Y. et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int. Immunol. 20, 791–800 (2008).
Xu, D. et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc. Natl Acad. Sci. USA 105, 10913–10918 (2008).
Verri, W. A. Jr. et al. IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. Proc. Natl Acad. Sci. USA 105, 2723–2728 (2008).
Verri, W. A. Jr. et al. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann. Rheum. Dis. 69, 1697–1703 (2010).
Xu, D. et al. IL-33 exacerbates autoantibody-induced arthritis. J. Immunol. 184, 2620–2626 (2010).
Masamune, A. et al. Nuclear expression of interleukin-33 in pancreatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G821–G832 (2010).
Lohning, M. et al. T1/ST2 is preferentially expressed on murine TH2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for TH2 effector function. Proc. Natl Acad. Sci. USA 95, 6930–6935 (1998).
Coyle, A. J. et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J. Exp. Med. 190, 895–902 (1999).
Hoshino, K. et al. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J. Exp. Med. 190, 1541–1548 (1999).
Townsend, M. J., Fallon, P. G., Matthews, D. J., Jolin, H. E. & McKenzie, A. N. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J. Exp. Med. 191, 1069–1076 (2000).
Senn, K. A. et al. T1-deficient and T1-Fc-transgenic mice develop a normal protective TH2-type immune response following infection with Nippostrongylus brasiliensis. Eur. J. Immunol. 30, 1929–1938 (2000).
Mangan, N. E., Dasvarma, A., McKenzie, A. N. & Fallon, P. G. T1/ST2 expression on TH2 cells negatively regulates allergic pulmonary inflammation. Eur. J. Immunol. 37, 1302–1312 (2007).
Zhiguang, X. et al. Over-expression of IL-33 leads to spontaneous pulmonary inflammation in mIL-33 transgenic mice. Immunol. Lett. 131, 159–165 (2010).
Leung, B. P., Xu, D., Culshaw, S., McInnes, I. B. & Liew, F. Y. A novel therapy of murine collagen-induced arthritis with soluble T1/ST2. J. Immunol. 173, 145–150 (2004).
Saleh, H. et al. Interleukin-33, a target of parathyroid hormone and oncostatin M, increases osteoblastic matrix mineral deposition and inhibits osteoclast formation in vitro. Endocrinology doi:10.1210/en.2010–1268.
Saidi, S. et al. IL-33 is expressed in human osteoblasts, but has no direct effect on bone remodeling. Cytokine 53, 347–354 (2011).
Jones, L. A. et al. IL-33 receptor (T1/ST2) signalling is necessary to prevent the development of encephalitis in mice infected with Toxoplasma gondii. Eur. J. Immunol. 40, 426–436 (2010).
Wieland, C. W., van der Windt, G. J., Florquin, S., McKenzie, A. N. & van der Poll, T. ST2 deficient mice display a normal host defense against pulmonary infection with Mycobacterium tuberculosis. Microbes Infect. 11, 524–530 (2009).
Alves-Filho, J. C. et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat. Med. 16, 708–712 (2010).
Choi, Y. S. et al. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood 114, 3117–3126 (2009).
Miller, A. M. et al. IL-33 reduces the development of atherosclerosis. J. Exp. Med. 205, 339–346 (2008).
McLaren, J. E. et al. IL-33 reduces macrophage foam cell formation. J. Immunol. 185, 1222–1229 (2010).
Seki, K. et al. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ. Heart Fail. 2, 684–691 (2009).
Sakashita, M. et al. Association of serum interleukin-33 level and the interleukin-33 genetic variant with Japanese cedar pollinosis. Clin. Exp. Allergy 38, 1875–1881 (2008).
Mu, R. et al. Elevated serum interleukin 33 is associated with autoantibody production in patients with rheumatoid arthritis. J. Rheumatol. 37, 2006–2013 (2010).
Talabot-Ayer, D. et al. Distinct serum and synovial fluid interleukin (IL)-33 levels in rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Joint Bone Spine doi:10.1016/j.jbspin.2011.02.011.
Mok, M. Y. et al. Serum levels of IL-33 and soluble ST2 and their association with disease activity in systemic lupus erythematosus. Rheumatology (Oxford) 49, 520–527 (2010).
Yang, Z., Liang, Y., Xi, W., Li, C. & Zhong, R. Association of increased serum IL-33 levels with clinical and laboratory characteristics of systemic lupus erythematosus in Chinese population. Clin. Exp. Med. doi:10.1007/s10238-010-0115–4.
Manetti, M. et al. The IL1-like cytokine IL33 and its receptor ST2 are abnormally expressed in the affected skin and visceral organs of patients with systemic sclerosis. Ann. Rheum. Dis. 69, 598–605 (2010).
Sahlander, K., Larsson, K. & Palmberg, L. Increased serum levels of soluble ST2 in birch pollen atopics and individuals working in laboratory animal facilities. J. Occup. Environ. Med. 52, 214–218 (2010).
Oshikawa, K. et al. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am. J. Respir. Crit. Care Med. 164, 277–281 (2001).
Brunner, M. et al. Increased levels of soluble ST2 protein and IgG1 production in patients with sepsis and trauma. Intensive Care Med. 30, 1468–1473 (2004).
Shimpo, M. et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation 109, 2186–2190 (2004).
Shah, R. V. & Januzzi, J. L. Jr. ST2: a novel remodeling biomarker in acute and chronic heart failure. Curr. Heart Fail. Rep. 7, 9–14 (2010).
Hoogerwerf, J. J. et al. Soluble ST2 plasma concentrations predict mortality in severe sepsis. Intensive Care Med. 36, 630–637 (2010).
Oppenheim, J. J. & Yang, D. Alarmins: chemotactic activators of immune responses. Curr. Opin. Immunol. 17, 359–365 (2005).
Acknowledgements
This work was supported by the Swiss National Science Foundation grants 320000-120319 (to G. Palmer) and 320000-119728 (to C. Gabay), the Rheumasearch Foundation, the de Reuter Foundation and the Institute of Arthritis Research.
Author information
Authors and Affiliations
Contributions
G. Palmer and C. Gabay contributed equally to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Palmer, G., Gabay, C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol 7, 321–329 (2011). https://doi.org/10.1038/nrrheum.2011.53
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2011.53
This article is cited by
-
Serum interleukin-33 and soluble suppression of tumorigenicity 2 in pediatric leukemia with febrile neutropenia
European Journal of Pediatrics (2024)
-
Hypo-osmotic stress induces the epithelial alarmin IL-33 in the colonic barrier of ulcerative colitis
Scientific Reports (2022)
-
IL-33/ST2 axis is involved in disease progression in the spleen during Leishmania donovani infection
Parasites & Vectors (2020)
-
IL-33 deficiency protects mice from DSS-induced experimental colitis by suppressing ILC2 and Th17 cell responses
Inflammation Research (2020)
-
Modeling of the immune response in the pathogenesis of solid tumors and its prognostic significance
Cellular Oncology (2020)