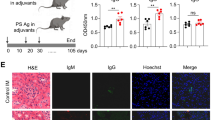
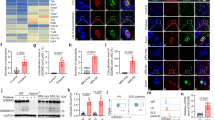
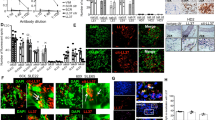
Abstract
Nucleic acids represent the main source of autoantigens in systemic lupus erythematosus (SLE). DNA and RNA can exit the cell during cell death and, in the extracellular space, can be immunostimulatory. Also extracellularly, DNA and RNA can be incorporated into microparticles (MPs)—small, membrane-bound vesicles released from dying cells by blebbing. We suggest that MPs display autoantigens, such as RNA and DNA, in a highly immunostimulatory manner, enabling them to function as autoadjuvants. In the bone marrow, nucleic-acid-containing MP autoadjuvants might induce B-cell tolerance, whereas in the periphery, they might stimulate mature B cells that have escaped central tolerance. Indeed, because MP autoadjuvants can trigger several receptors, they could effectively provide apoptotic or activating signals to B cells. We would therefore advance the idea that a model for SLE based on MP autoadjuvants can provide a new paradigm to elucidate the mechanisms by which DNA and RNA affect the immune system and critically influence B-cell fate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ardoin, S. P. & Pisetsky, D. S. Developments in the scientific understanding of lupus. Arthritis Res. Ther. 10, 218 (2008).
Kirou, K. A. et al. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 52, 1491–1503 (2005).
Ronnblom, L., Eloranta, M. L. & Alm, G. V. The type I interferon system in systemic lupus erythematosus. Arthritis Rheum. 54, 408–420 (2006).
Deane, J. A. & Bolland, S. Nucleic acid-sensing TLRs as modifiers of autoimmunity. J. Immunol. 177, 6573–6578 (2006).
Martin, D. A. & Elkon, K. B. Intracellular mammalian DNA stimulates myeloid dendritic cells to produce type I interferons predominantly through a toll-like receptor 9-independent pathway. Arthritis Rheum. 54, 951–962 (2006).
Lande, R. et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569 (2007).
Beyer, C. & Pisetsky, D. S. The role of microparticles in the pathogenesis of rheumatic diseases. Nat. Rev. Rheumatol. 6, 21–29 (2010).
Pisetsky, D. S. Immune activation by bacterial DNA: a new genetic code. Immunity 5, 303–310 (1996).
Krieg, A. M. et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 6, 546–549 (1995).
Yasuda, K. et al. Requirement for DNA CpG content in TLR9-dependent dendritic cell activation induced by DNA-containing immune complexes. J. Immunol. 183, 3109–3117 (2009).
Xiao, T. Innate immune recognition of nucleic acids. Immunol. Res. 43, 98–108 (2009).
Vallin, H., Perers, A., Alm, G. V. & Ronnblom, L. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-alpha inducer in systemic lupus erythematosus. J. Immunol. 163, 6306–6313 (1999).
Boule, M. W. et al. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J. Exp. Med. 199, 1631–1640 (2004).
Tian, J. et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 8, 487–496 (2007).
Yasuda, K. et al. Murine dendritic cell type I IFN production induced by human IgG-RNA immune complexes is IFN regulatory factor (IRF)5 and IRF7 dependent and is required for IL-6 production. J. Immunol. 178, 6876–6885 (2007).
Jiang, W., Reich, I. C. & Pisetsky, D. S. Mechanisms of activation of the RAW264.7 macrophage cell line by transfected mammalian DNA. Cell. Immunol. 229, 31–40 (2004).
Jiang, W. & Pisetsky, D. S. The induction of HMGB1 release from RAW 264.7 cells by transfected DNA. Mol. Immunol. 45, 2038–2044 (2008).
Marques, J. T. et al. A structural basis for discriminating between self and nonself double-stranded RNAs in mammalian cells. Nat. Biotechnol. 24, 559–565 (2006).
Fernandes-Alnemri, T., Yu, J.-W., Datta, P., Wu, J. & Alnemri, E. S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–515 (2009).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Crow, Y. J. & Rehwinkel, J. Aicardi-Goutieres syndrome and related phenotypes: linking nucleic acid metabolism with autoimmunity. Hum. Mol. Gen. 18, R130–R136 (2009).
Halicka, H. D., Bedner, E. & Darzynkiewicz, Z. Segregation of RNA and separate packaging of DNA and RNA in apoptotic bodies during apoptosis. Exp. Cell Res. 260, 248–256 (2000).
Ratajczak, J., Wysoczynski, M., Hayek, F., Janowska-Wieczorek, A. & Ratajczak, M. Z. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20, 1487–1495 (2006).
Piccin, A., Murphy, W. G. & Smith, O. P. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 21, 157–171 (2007).
Hasselmann, D. O., Rappl, G., Tilgen, W. & Reinhold, U. Extracellular tyrosinase mRNA within apoptotic bodies is protected from degradation in human serum. Clin. Chem. 47, 1488–1489 (2001).
Ng, E. K. et al. Presence of filterable and nonfilterable mRNA in the plasma of cancer patients and healthy individuals. Clin. Chem. 48, 1212–1217 (2002).
Reich, C. F. & Pisetsky, D. S. The content of DNA and RNA in microparticles released by Jurkat and HL-60 cells undergoing in vitro apoptosis. Exp. Cell Res. 315, 760–768 (2009).
De Gregorio, E., D'Oro, U. & Wack, A. Immunology of TLR-independent vaccine adjuvants. Curr. Opin. Immunol. 21, 339–345 (2009).
Yurasov, S. et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 201, 703–711 (2005).
Jacobi, A. M., Zhang, J., Mackay, M., Aranow, C. & Diamond, B. Phenotypic characterization of autoreactive B cells—checkpoints of B cell tolerance in patients with systemic lupus erythematosus. PLoS ONE 4, e5776 (2009).
Tussiwand, R., Bosco, N., Ceredig, R. & Rolink, A. G. Tolerance checkpoints in B-cell development: Johnny B good. Eur. J. Immunol. 39, 2317–2324 (2009).
Lee, J., Kuchen, S., Fischer, R., Chang, S. & Lipsky, P. E. Identification and characterization of a human CD5+ pre-naive B cell population. J. Immunol. 182, 4116–4126 (2009).
Alexander, T. et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood 113, 214–223 (2009).
Munoz, L. E. et al. SLE-—a disease of clearance deficiency? Rheumatology (Oxford) 44, 1101–1107 (2005).
Ogden, C. A. & Elkon, K. B. Role of complement and other innate immune mechanisms in the removal of apoptotic cells. Curr. Dir. Autoimmun. 9, 120–142 (2006).
Schiller, M. et al. Autoantigens are translocated into small apoptotic bodies during early stages of apoptosis. Cell Death Differ. 15, 183–191 (2007).
Carrasco, Y. R. & Batista, F. D. B cell recognition of membrane-bound antigen: an exquisite way of sensing ligands. Curr. Opin. Immunol. 18, 286–291 (2006).
Tolar, P., Sohn, H. W., Liu, W. & Pierce, S. K. The molecular assembly and organization of signaling active B-cell receptor oligomers. Immunol. Rev. 232, 34–52 (2009).
Gould, S. J., Booth, A. M. & Hildreth, J. E. The Trojan exosome hypothesis. Proc. Natl Acad. Sci. USA 100, 10592–10597 (2003).
Christensen, S. R. et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25, 417–428 (2006).
Nickerson, K. M. et al. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J. Immunol. 184, 1840–1848 (2010).
Krauss, S. W. et al. Nuclear substructure reorganization during late-stage erythropoiesis is selective and does not involve caspase cleavage of major nuclear substructural proteins. Blood 106, 2200–2205 (2005).
Napirei, M. et al. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat. Genet. 25, 177–181 (2000).
Jiang, N., Reich, C. F. & Pisetsky, D. S. Role of macrophages in the generation of circulating blood nucleosomes from dead and dying cells. Blood 102, 2243–2250 (2003).
Jungel, A. et al. Microparticles stimulate the synthesis of prostaglandin E(2) via induction of cyclooxygenase 2 and microsomal prostaglandin E synthase 1. Arthritis Rheum. 56, 3564–3574 (2007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Pisetsky, D., Lipsky, P. Microparticles as autoadjuvants in the pathogenesis of SLE. Nat Rev Rheumatol 6, 368–372 (2010). https://doi.org/10.1038/nrrheum.2010.66
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2010.66
This article is cited by
-
Characterization of cell-derived microparticles in synovial fluid and plasma of patients with rheumatoid arthritis
Rheumatology International (2019)
-
Circulating DNA in rheumatoid arthritis: pathological changes and association with clinically used serological markers
Arthritis Research & Therapy (2017)
-
Microparticles: a critical component in the nexus between inflammation, immunity, and thrombosis
Seminars in Immunopathology (2011)
-
Impaired clearance of apoptotic cells in germinal centers: implications for loss of B cell tolerance and induction of autoimmunity
Immunologic Research (2011)