Abstract
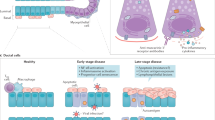
Sjögren's syndrome (SS), a chronic autoimmune disorder, particularly compromises the function of exocrine glands. The involvement of these glands is characterized by focal, mononuclear cell infiltrates that surround the ducts and replace the secretory units. The pathogenetic mechanisms of this autoimmune exocrinopathy have not been fully elucidated. Immunologically-activated or apoptotic glandular epithelial cells that expose autoantigens in genetically predisposed individuals might drive autoimmune-mediated tissue injury. Alterations in several immune mediators, such as upregulation of type I interferon-regulated genes, abnormal expression of B-cell-activating factor and activation of the interleukin-23–type 17 T-helper cell pathway, have been reported. Extension of the pathological process that affects the exocrine glands into periepithelial and extraepithelial tissue can cause a considerable percentage of patients to exhibit systemic findings that involve the lungs, liver or kidneys. These manifestations develop as a result of lymphocytic invasion or an immune-complex-mediated process, or both, and present as skin vasculitis coupled with peripheral neuropathy or glomerulonephritis (or both). Patients with systemic extraepithelial manifestations display low serum levels of the complement component C4 and mixed type II cryoglobulins, and show an increased risk of developing non-Hodgkin lymphoma, thereby reflecting an overall worse prognosis with higher mortality rates than those without extraepithelial manifestations.
Key Points
-
At the molecular and cellular levels, epithelial cells have an important role in the initiation and perpetuation of autoimmune lesions in Sjögren's syndrome (SS)
-
Antigen presentation, apoptosis, chemokine production or germinal center formation lie at the center of SS pathogenesis; epithelial cells have a key role in all these processes
-
Alterations in a number of immune mediators contribute to chronic immune dysregulation
-
These changes include: upregulation of type I-interferon-regulated genes; abnormal expression of B-cell-activating factor; and activation of the interleukin-23–T-helper type 17 cell pathway
-
Among autoimmune diseases, SS displays the highest incidence of malignant lymphoproliferative disorders
-
Severe involvement of exocrine glands, vasculitis, low C4 levels and cryoglobulinemia at diagnosis identify specific SS patients with a high risk of lymphoma development and therefore high mortality rates
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Anaya, J. M. & Talal, N. In Arthritis and Allied Conditions: a Textbook of Rheumatology 13th edn (ed. Koopman, W. J.) 1561–1580 (Williams & Wilkins, Baltimore, 1997).
Alamanos, Y. et al. Epidemiology of primary Sjögren's syndrome in north-west Greece, 1982–2003. Rheumatology (Oxford) 45, 187–191 (2006).
Trontzas, P. I. & Andrianakos, A. A. Sjogren's syndrome: a population based study of prevalence in Greece. The ESORDIG study. Ann. Rheum. Dis. 64, 1240–1241 (2005).
Bowman, S. J., Ibrahim, G. H., Holmes, G., Hamburger, J. & Ainsworth, J. R. Estimating the prevalence among Caucasian women of primary Sjögren's syndrome in two general practices in Birmingham, UK. Scand. J. Rheumatol. 33, 39–43 (2004).
Delaleu, N., Jonsson, M. V., Appel, S. & Jonsson, R. New concepts in the pathogenesis of Sjögren's syndrome. Rheum. Dis. Clin. North Am. 34, 833–845 (2008).
Venables, P. J. Sjögren's syndrome. Best Pract. Res. Clin. Rheumatol. 18, 313–329 (2004).
Ramos-Casals, M. et al. SS-HCV Study Group. Sjögren syndrome associated with hepatitis C virus: a multicenter analysis of 137 cases. Medicine (Baltimore) 84, 81–89 (2005).
Moutsopoulos, H. M. Sjögren's syndrome: autoimmune epithelitis. Clin. Immunol. Immunopathol. 72, 162–165 (1994).
Papiris, S. A. et al. Lung involvement in primary Sjögren's syndrome is mainly related to the small airway disease. Ann. Rheum. Dis. 58, 61–64 (1999).
Tu, W. H., Shearn, M. A., Lee, J. C. & Hopper, J. Jr. Interstitial nephritis in Sjögren's syndrome. Ann. Intern. Med. 69, 1163–1170 (1968).
Skopouli, F. N., Barbatis, C. & Moutsopoulos, H. M. Liver involvement in primary Sjögren's syndrome. Br. J. Rheumatol. 33, 745–748 (1994).
Ramos-Casals, M. et al. Cutaneous vasculitis in primary Sjögren syndrome: classification and clinical significance of 52 patients. Medicine (Baltimore) 83, 96–106 (2004).
Talal, N., Zisman, E. & Schur, P. H. Renal tubular acidosis, glomerulonephritis and immunologic factors in Sjögren's syndrome. Arthritis Rheum. 11, 774–786 (1968).
Tsokos, M., Lazarou, S. A. & Moutsopoulos, H. M. Vasculitis in primary Sjögren's syndrome. Histologic classification and clinical presentation. Am. J. Clin. Pathol. 88, 26–31 (1987).
Skopouli, F. N., Dafni, U., Ioannidis, J. P. & Moutsopoulos, H. M. Clinical evolution, and morbidity and mortality of primary Sjogren's syndrome. Semin. Arthritis Rheum. 29, 296–304 (2000).
Voulgarelis, M., Dafni, U. G., Isenberg, D. A. & Moutsopoulos, H. M. Malignant lymphoma in primary Sjögren's syndrome: a multicenter, retrospective, clinical study by the European Concerted Action on Sjögren's Syndrome. Arthritis Rheum. 42, 1765–1772 (1999).
Tzioufas, A. G., Boumba, D. S., Skopouli, F. N. & Moutsopoulos, H. M. Mixed monoclonal cryoglobulinemia and monoclonal rheumatoid factor cross-reactive idiotypes as predictive factors for the development of lymphoma in primary Sjögren's syndrome. Arthritis Rheum. 39, 767–772 (1996).
Ioannidis, J. P., Vassiliou, V. A. & Moutsopoulos, H. M. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjogren's syndrome. Arthritis Rheum. 46, 741–747 (2002).
Katsifis, G. E., Moutsopoulos, N. M. & Wahl, S. M. T lymphocytes in Sjögren's syndrome: contributors to and regulators of pathophysiology. Clin. Rev. Allergy Immunol. 32, 252–264 (2007).
Manoussakis, M. N. et al. Rates of infiltration by macrophages and dendritic cells and expression of interleukin-18 and interleukin-12 in the chronic inflammatory lesions of Sjögren's syndrome: correlation with certain features of immune hyperactivity and factors associated with high risk of lymphoma development. Arthritis Rheum. 56, 3977–3988 (2007).
Bolstad, A. I. & Jonsson, R. Genetic aspects of Sjögren's syndrome. Arthritis Res. 4, 353–359 (2002).
Gottenberg, J. E. et al. In primary Sjögren's syndrome, HLA class II is associated exclusively with autoantibody production and spreading of the autoimmune response. Arthritis Rheum. 48, 2240–2245 (2003).
Miyagawa, S. et al. Polymorphisms of HLA class II genes and autoimmune responses to Ro/SS-A–La/SS-B among Japanese subjects. Arthritis Rheum. 41, 927–934 (1998).
Miceli-Richard, C. et al. Association of an IRF5 gene functional polymorphism with Sjögren's syndrome. Arthritis Rheum. 56, 3989–3994 (2007).
Nordmark, G. et al. Additive effects of the major risk alleles of IRF5 and STAT4 in primary Sjögren's syndrome. Genes Immun. 10, 68–76 (2009).
Shim, G. J. et al. Aromatase-deficient mice spontaneously develop a lymphoproliferative autoimmune disease resembling Sjogren's syndrome. Proc. Natl Acad. Sci. USA 101, 12628–12633 (2004).
Ishimaru, N. et al. Expression of the retinoblastoma protein RbAp48 in exocrine glands leads to Sjögren's syndrome-like autoimmune exocrinopathy. J. Exp. Med. 205, 2915–2927 (2008).
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003).
Pflugfelder, S. C. et al. Epstein–Barr virus and the lacrimal gland pathology of Sjögren's syndrome. Am. J. Pathol. 143, 49–64 (1993).
Green, J. E., Hinrichs, S. H., Vogel, J. & Jay, G. Exocrinopathy resembling Sjögren's syndrome in HTLV-1 tax transgenic mice. Nature 341, 72–74 (1989).
Saito, I., Servenius, B., Compton, T. & Fox, R. I. Detection of Epstein–Barr virus DNA by polymerase chain reaction in blood and tissue biopsies from patients with Sjögren's syndrome. J. Exp. Med. 169, 2191–2198 (1989).
Mariette, X., Gozlan, J., Clerc, D., Bisson, M. & Morinet, F. Detection of Epstein–Barr virus DNA by in situ hybridization and polymerase chain reaction in salivary gland biopsy specimens from patients with Sjögren's syndrome. Am. J. Med. 90, 286–294 (1991).
Inoue, H. et al. Possible involvement of EBV-mediated alpha-fodrin cleavage for organ-specific autoantigen in Sjögren's syndrome. J. Immunol. 166, 5801–5809 (2001).
Nagata, Y. et al. Activation of Epstein–Barr virus by saliva from Sjögren's syndrome patients. Immunology 111, 223–229 (2004).
Yamaoka, K., Miyasaka, N. & Yamamoto, K. Possible involvement of Epstein–Barr virus in polyclonal B cell activation in Sjögren's syndrome. Arthritis Rheum. 31, 1014–1021 (1988).
Triantafyllopoulou, A., Tapinos, N. & Moutsopoulos, H. M. Evidence for coxsackievirus infection in primary Sjögren's syndrome. Arthritis Rheum. 50, 2897–2902 (2004).
Spachidou, M. P. et al. Expression of functional Toll-like receptors by salivary gland epithelial cells: increased mRNA expression in cells derived from patients with primary Sjögren's syndrome. Clin. Exp. Immunol. 147, 497–503 (2007).
Gottenberg, J. E. et al. Failure to confirm coxsackievirus infection in primary Sjögren's syndrome. Arthritis Rheum. 54, 2026–2028 (2006).
Cha, S., Peck, A. B. & Humphreys-Beher, M. G. Progress in understanding autoimmune exocrinopathy using the non-obese diabetic mouse: an update. Crit. Rev. Oral Biol. Med. 13, 5–16 (2002).
McArthur, C. P., Daniels, P. J., Kragel, P., Howard, P. F. & Julian, L. Sjögren's syndrome salivary gland immunopathology: increased laminin expression precedes lymphocytic infiltration. J. Autoimmun. 10, 59–65 (1997).
Goicovich, E. et al. Enhanced degradation of proteins of the basal lamina and stroma by matrix metalloproteinases from the salivary glands of Sjögren's syndrome patients: correlation with reduced structural integrity of acini and ducts. Arthritis Rheum. 48, 2573–2584 (2003).
Molina, C. et al. Basal lamina disorganisation of the acini and ducts of labial salivary glands from patients with Sjögren's syndrome: association with mononuclear cell infiltration. Ann. Rheum. Dis. 65, 178–183 (2006).
Pérez, P. et al. Differential expression of matrix metalloproteinases in labial salivary glands of patients with primary Sjögren's syndrome. Arthritis Rheum. 43, 2807–2817 (2000).
Aziz, K. E., McCluskey, P. J., Montanaro, A. & Wakefield, D. Vascular endothelium and lymphocyte adhesion molecules in minor salivary glands of patients with Sjögren's syndrome. J. Clin. Lab. Immunol. 37, 39–49 (1992).
Xanthou, G. et al. “Lymphoid” chemokine messenger RNA expression by epithelial cells in the chronic inflammatory lesion of the salivary glands of Sjögren's syndrome patients: possible participation in lymphoid structure formation. Arthritis Rheum. 44, 408–418 (2001).
Moutsopoulos, H. M. et al. HLA-DR expression by labial minor salivary gland tissues in Sjögren's syndrome. Ann. Rheum. Dis. 45, 677–683 (1986).
Manoussakis, M. N. et al. Expression of B7 costimulatory molecules by salivary gland epithelial cells in patients with Sjögren's syndrome. Arthritis Rheum. 42, 229–239 (1999).
Matsumura, R. et al. Glandular and extraglandular expression of costimulatory molecules in patients with Sjögren's syndrome. Ann. Rheum. Dis. 60, 473–482 (2001).
Manoussakis, M. N. & Kapsogeorgou, E. K. The role of epithelial cells in the pathogenesis of Sjögren's syndrome. Clin. Rev. Allergy Immunol. 32, 225–230 (2007).
Tsunawaki, S. et al. Possible function of salivary gland epithelial cells as nonprofessional antigen-presenting cells in the development of Sjögren's syndrome. J. Rheumatol. 29, 1884–1896 (2002).
Dimitriou, I. D., Kapsogeorgou, E. K., Moutsopoulos, H. M. & Manoussakis, M. N. CD40 on salivary gland epithelial cells: high constitutive expression by cultured cells from Sjögren's syndrome patients indicating their intrinsic activation. Clin. Exp. Immunol. 127, 386–392 (2002).
Lavie, F. et al. Expression of BAFF (BLyS) in T cells infiltrating labial salivary glands from patients with Sjögren's syndrome. J. Pathol. 202, 496–502 (2004).
Ohlsson, M., Szodoray, P., Loro, L. L., Johannessen, A. C. & Jonsson, R. CD40, CD154, Bax and Bcl-2 expression in Sjögren's syndrome salivary glands: a putative anti-apoptotic role during its effector phases. Scand. J. Immunol. 56, 561–571 (2002).
Mitsias, D. I., Kapsogeorgou, E. K. & Moutsopoulos, H. M. The role of epithelial cells in the initiation and perpetuation of autoimmune lesions: lessons from Sjögren's syndrome (autoimmune epithelitis). Lupus 15, 255–261 (2006).
Jonsson, R., Gordon, T. P. & Konttinen, Y. T. Recent advances in understanding molecular mechanisms in the pathogenesis and antibody profile of Sjögren's syndrome. Curr. Rheumatol. Rep. 5, 311–316 (2003).
Vogelsang, P., Jonsson, M. V., Dalvin, S. T. & Appel, S. Role of dendritic cells in Sjögren's syndrome. Scand. J. Immunol. 64, 219–226 (2006).
Kong, L. et al. Bcl-2 family expression in salivary glands from patients with primary Sjögren's syndrome: involvement of Bax in salivary gland destruction. Clin. Immunol. Immunopathol. 88, 133–141 (1998).
Kapsogeorgou, E. K., Abu-Helu, R. F., Moutsopoulos, H. M. & Manoussakis, M. N. Salivary gland epithelial cell exosomes: A source of autoantigenic ribonucleoproteins. Arthritis Rheum. 52, 1517–1521 (2005).
Ittah, M. et al. B-cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjögren's syndrome. Arthritis Res. Ther. 8, R51 (2006).
Ittah, M. et al. B-cell-activating factor expressions in salivary epithelial cells after dsRNA virus infection depends on RNA-activated protein kinase activation. Eur. J. Immunol. 39, 1271–1279 (2009).
Båve, U. et al. Activation of the type I interferon system in primary Sjögren's syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 52, 1185–1195 (2005).
Gottenberg, J. E. et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren's syndrome. Proc. Natl Acad. Sci. USA 103, 2770–2775 (2006).
Ma-Krupa, W. et al. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J. Exp. Med. 199, 173–183 (2004).
Deshmukh, U. S., Nandula, S. R., Thimmalapura, P. R., Scindia, Y. M. & Bagavant, H. Activation of innate immune responses through Toll-like receptor 3 causes a rapid loss of salivary gland function. J. Oral Pathol. Med. 38, 42–47 (2009).
Robinson, C. P. et al. Characterization of the changing lymphocyte populations and cytokine expression in the exocrine tissues of autoimmune NOD mice. Autoimmunity 27, 29–44 (1998).
Robinson, C. P. et al. Infiltrating lymphocyte populations and cytokine production in the salivary and lacrimal glands of autoimmune NOD mice. Adv. Exp. Med. Biol. 438, 493–497 (1998).
Kolkowski, E. C. et al. TH1 predominance and perforin expression in minor salivary glands from patients with primary Sjögren's syndrome. J. Autoimmun. 13, 155–162 (1999).
Mitsias, D. I. et al. The TH1/TH2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren's syndrome. Clin. Exp. Immunol. 128, 562–568 (2002).
Nguyen, C. Q., Hu, M. H., Li, Y., Stewart, C. & Peck, A. B. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren's syndrome: findings in humans and mice. Arthritis Rheum. 58, 734–743 (2008).
Bettelli, E., Korn, T., Oukka, M. & Kuchroo, V. K. Induction and effector functions of TH17 cells. Nature 453, 1051–1057 (2008).
Espinosa, A. et al. Loss of the lupus autoantigen Ro52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23–TH17 pathway. J. Exp. Med. 206, 1661–1671 (2009).
Katsifis, G. E., Rekka, S., Moutsopoulos, N. M., Pillemer, S. & Wahl, S. M. Systemic and local interleukin 17 and linked cytokines associated with Sjögren's syndrome immunopathogenesis. Am. J. Pathol. 175, 1167–1177 (2009).
Christodoulou, M. I., Kapsogeorgou, E. K., Moutsopoulos, N. M. & Moutsopoulos, H. M. Foxp3+ T-regulatory cells in Sjögren's syndrome: correlation with the grade of the autoimmune lesion and certain adverse prognostic factors. Am. J. Pathol. 173, 1389–1396 (2008).
Christodoulou, M. I., Kapsogeorgou, E. K. & Moutsopoulos, H. M. Characteristics of the minor salivary gland infiltrates in Sjögren's syndrome. J. Autoimmun. 34, 400–407 (2010).
Zintzaras, E., Voulgarelis, M. & Moutsopoulos, H. M. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch. Intern. Med. 165, 2337–2344 (2005).
Fox, R. I. Sjögren's syndrome. Lancet 366, 321–331 (2005).
Salomonsson, S. et al. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren's syndrome. Arthritis Rheum. 48, 3187–3201 (2003).
Hansen, A., Lipsky, P. E. & Dörner, T. B cells in Sjögren's syndrome: indications for disturbed selection and differentiation in ectopic lymphoid tissue. Arthritis Res. Ther. 9, 218 (2007).
Jonsson, M. V., Skarstein, K., Jonsson, R. & Brun, J. G. Serological implications of germinal center-like structures in primary Sjögren's syndrome. J. Rheumatol. 34, 2044–2049 (2007).
Le Pottier, L. et al. Ectopic germinal centers are rare in Sjögren's syndrome salivary glands and do not exclude autoreactive B cells. J. Immunol. 182, 3540–3547 (2009).
Szodoray, P. et al. Distinct profiles of Sjögren's syndrome patients with ectopic salivary gland germinal centers revealed by serum cytokines and BAFF. Clin. Immunol. 117, 168–176 (2005).
Daridon, C. et al. Aberrant expression of BAFF by B lymphocytes infiltrating the salivary glands of patients with primary Sjögren's syndrome. Arthritis Rheum. 56, 1134–1144 (2007).
Sutherland, A. P., Mackay, F. & Mackay, C. R. Targeting BAFF: immunomodulation for autoimmune diseases and lymphomas. Pharmacol. Ther. 112, 774–786 (2006).
Mariette, X. et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjögren's syndrome. Ann. Rheum. Dis. 62, 168–171 (2003).
Groom, J. et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren's syndrome. J. Clin. Invest. 109, 59–68 (2002).
Cheema, G. S., Roschke, V., Hilbert, D. M. & Stohl, W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 44, 1313–1319 (2001).
Sellam, J. et al. Decreased B cell activating factor receptor expression on peripheral lymphocytes associated with increased disease activity in primary Sjögren's syndrome and systemic lupus erythematosus. Ann. Rheum. Dis. 66, 790–797 (2007).
Jacobi, A. M. et al. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 48, 1332–1342 (2003).
Hansen, A. et al. Diminished peripheral blood memory B cells and accumulation of memory B cells in the salivary glands of patients with Sjögren's syndrome. Arthritis Rheum. 46, 2160–2171 (2002).
DiGiuseppe, J. A., Corio, R. L. & Westra, W. H. Lymphoid infiltrates of the salivary glands: pathology, biology and clinical significance. Curr. Opin. Oncol. 8, 232–237 (1996).
Hansen, A., Lipsky, P. E. & Dörner, T. B-cell lymphoproliferation in chronic inflammatory rheumatic diseases. Nat. Clin. Pract. Rheumatol. 3, 561–569 (2007).
Tapinos, N. I., Polihronis, M. & Moutsopoulos, H. M. Lymphoma development in Sjögren's syndrome: novel p53 mutations. Arthritis Rheum. 42, 1466–1472 (1999).
Kassan, S. S. et al. Increased risk of lymphoma in sicca syndrome. Ann. Inter. Med. 89, 888–892 (1978).
Baimpa, E., Dahabreh, I. J., Voulgarelis, M. & Moutsopoulos, H. M. Hematologic manifestations and predictors of lymphoma development in primary Sjögren syndrome: clinical and pathophysiologic aspects. Medicine (Baltimore) 88, 284–293 (2009).
Mariette, X. Lymphomas complicating Sjögren's syndrome and hepatitis C virus infection may share a common pathogenesis: chronic stimulation of rheumatoid factor B cells. Ann. Rheum. Dis. 60, 1007–1010 (2001).
Anderson, L. G. & Talal, N. The spectrum of benign to malignant lymphoproliferation in Sjogren's syndrome. Clin. Exp. Immunol. 10, 199–221 (1972).
Anaya, J. M., McGuff, H. S., Banks, P. M. & Talal, N. Clinicopathological factors relating malignant lymphoma with Sjogren's syndrome. Semin. Arthritis Rheum. 25, 337–346 (1996).
Valesini, G. et al. Differential risk of non-Hodgkin's lymphoma in Italian patients with primary Sjogren's syndrome. J. Rheumatol. 24, 2376–2380 (1997).
Theander, E. et al. Lymphoma and other malignancies in primary Sjogren's syndrome: A cohort study on cancer incidence and lymphoma predictors. Ann. Rheum. Dis. 65, 796–803 (2006).
Voulgarelis, M., Tzioufas, A. G. & Moutsopoulos, H. M. Mortality in Sjögren's syndrome. Clin. Exp. Rheumatol. 26 (5 Suppl. 51), S66–S71 (2008).
Theander, E., Manthorpe, R. & Jacobsson, L. T. Mortality and causes of death in primary Sjögren's syndrome: a prospective cohort study. Arthritis Rheum. 50, 1262–1269 (2004).
Alamanos, Y. et al. Epidemiology of primary Sjögren's syndrome in north-west Greece, 1982–2003. Rheumatology (Oxford) 45, 187–191 (2006).
Brito-Zerón, P., Ramos-Casals, M., Bove, A., Sentis, J. & Font, J. Predicting adverse outcomes in primary Sjogren's syndrome: identification of prognostic factors. Rheumatology (Oxford) 46, 1359–1362 (2007).
Ramos-Casals, M. et al. Hypocomplementaemia as an immunological marker of morbidity and mortality in patients with primary Sjogren's syndrome. Rheumatology (Oxford) 44, 89–94 (2005).
Martens, P. B., Pillemer, S. R., Jacobsson, L. T., O'Fallon, W. M. & Matteson, E. L. Survivorship in a population based cohort of patients with Sjögren's syndrome, 1976–1992. J. Rheumatol. 26, 1296–1300 (1999).
Pertovaara, M., Pukkala, E., Laippala, P., Miettinen, A. & Pasternack, A. A longitudinal cohort study of Finnish patients with primary Sjögren's syndrome: clinical, immunological, and epidemiological aspects. Ann. Rheum. Dis. 60, 467–472 (2001).
Scalzi, L. V., Ballou, S. P., Park, J. Y., Redline, S. & Kirchner, H. L. Cardiovascular disease risk awareness in systemic lupus erythematosus patients. Arthritis Rheum. 58, 1458–1464 (2008).
Vaudo, G. et al. Precocious intima–media thickening in patients with primary Sjögren's syndrome. Arthritis Rheum. 52, 3890–3897 (2005).
Acknowledgements
We would like to thank Professor H. M. Moutsopoulos for his inspiration and guidance.
Author information
Authors and Affiliations
Contributions
M. Voulgarelis researched the data for the article, provided a substantial contribution to the discussion of content and wrote the article. A. G. Tzioufas reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Voulgarelis, M., Tzioufas, A. Pathogenetic mechanisms in the initiation and perpetuation of Sjögren's syndrome. Nat Rev Rheumatol 6, 529–537 (2010). https://doi.org/10.1038/nrrheum.2010.118
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2010.118
This article is cited by
-
Melatonin: a potential therapeutic approach for the management of primary Sjögren’s syndrome
Immunologic Research (2023)
-
Extracellular vesicle–encapsulated miR-10a-5p derived from MDSCs restrains germinal center B cells in experimental Sjögren’s syndrome
Immunologic Research (2023)
-
Sjögren’s syndrome with adult-onset Still’s disease: an overlap syndrome?
Rheumatology International (2022)
-
Diminished natural killer T-like cells correlates with aggravated primary Sjögren’s syndrome
Clinical Rheumatology (2022)
-
Olfactory ecto-mesenchymal stem cell-derived exosomes ameliorate murine Sjögren’s syndrome by modulating the function of myeloid-derived suppressor cells
Cellular & Molecular Immunology (2021)