Abstract
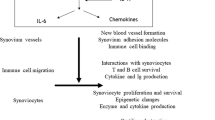
The discovery of interleukin (IL)-17 and its major cell source, the type 17 T-helper (TH17) lymphocyte, has been a major step in the understanding of erosive arthritis. This Review summarizes current knowledge of the role of IL-17 in this context derived from both animal models and studies in patients with rheumatoid arthritis. Evidence shows that IL-17 is present at sites of inflammatory arthritis and that, in synergistic interactions, it amplifies the inflammation induced by other cytokines, primarily tumor necrosis factor. In several animal models of arthritis, inhibition of IL-17 limits inflammation and joint erosion. Initial observations from phase I trials show that signs and symptoms of RA are significantly suppressed following treatment with anti-IL-17 antibodies, without notable adverse effects. The emergence of IL-17 blockade as a future therapy in rheumatoid arthritis is highlighted, along with the potential goals and limitations of this therapeutic approach.
Key Points
-
Interleukin 17 (IL-17) is present at sites of inflammatory arthritis
-
In synergistic interactions, IL-17 amplifies the inflammation induced by other cytokines, mostly tumor necrosis factor
-
Inhibition of IL-17 in several animal models of arthritis controls inflammation and joint destruction
-
IL-17 has a role in inflammatory arthritis phenotypes with a destructive disease course
-
In clinical trials, anti-IL-17 antibodies show efficacy in ameliorating the signs and symptoms of human rheumatoid arthritis, in line with preclinical results
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Yao, Z. et al. Human IL-17: a novel cytokine derived from T cells. J. Immunol. 155, 5483–5486 (1995).
Rouvier, E., Luciani, M. F., Mattei, M. G., Denizot, F. & Golstein, P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpes virus saimiri gene. J. Immunol. 150, 5445–5456 (1993).
Miossec, P. Interleukin-17 in fashion, at last: ten years after its description, its cellular source has been identified. Arthritis Rheum. 56, 2111–2115 (2007).
Fossiez, F. et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183, 2593–2603 (1996).
Kolls, J. K. & Lindén, A. Interleukin-17 family members and inflammation. Immunity 21, 467–476 (2004).
Iwakura, Y., Nakae, S., Saijo, S. & Ishigame, H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol. Rev. 226, 57–79 (2008).
Kotake, S. et al. IL-17 in synovial fluids from patients with RA is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 103, 1345–1352 (1999).
Koenders, M. I. et al. Induction of cartilage damage by overexpression of T cell IL-17A in experimental arthritis in mice deficient in IL-1. Arthritis Rheum. 52, 975–983 (2005).
Gaffen, S. L. An overview of IL-17 function and signaling. Cytokine 43, 402–407 (2008).
Toy, D. et al. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J. Immunol. 177, 36–39 (2006).
Maitra, A. et al. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc. Natl Acad. Sci. USA 104, 7507–7511 (2007).
Zrioual, S. et al. IL-17RA and IL-17RC receptors are essential for IL-17A-induced ELR+ CXC chemokine expression in synoviocytes and are overexpressed in rheumatoid blood. J. Immunol. 180, 655–663 (2008).
Aarvak, T., Chabaud, M., Miossec, P. & Natvig, J. B. IL-17 is produced by some proinflammatory TH1/TH0 cells but not by TH2 cells. J. Immunol. 162, 1246–1251 (1999).
Harrington, L. E. et al. IL-17 producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132 (2005).
Weaver, C. T., Harrington, L. E., Mangan, P. R., Gavrieli, M. & Murphy, K. M. TH17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 24, 677–688 (2006).
Bettelli, E., Korn, T., Oukka, M. & Kuchroo, V. K. Induction and effector functions of TH17 cells. Nature 453, 1051–1057 (2008).
Ivanov, B. S. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Aggarwal, S., Ghilardi, N., Xie, M. H., de Sauvage, F. J. & Gurney, A. L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278, 1910–1914 (2003).
Liang, S. C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by TH17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Geboes, L. et al. Proinflammatory role of the TH17 cytokine IL-22 in collagen-induced arthritis in C57Bl/6 mice. Arthritis Rheum. 60, 390–395 (2009).
Lubberts, E. et al. IL-1 independent role of IL-17 in synovial inflammation and joint destruction during collagen induced arthritis. J. Immunol. 167, 1004–1013 (2001).
Miossec, P. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 48, 594–601 (2003).
Sato, K. et al. TH17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 203, 2673–2682 (2006).
Koenders, M. I., Joosten, L. A. & van den Berg, W. B. Potential new targets in arthritis therapy: interleukin (IL)-17 and its relations to tumour necrosis factor and IL-1 in experimental arthritis. Ann. Rheum. Dis. 65 (Suppl. 3), iii29–iii33 (2006).
Koenders, M. I. et al. Interleukin-17 acts independently of TNF-α under arthritic conditions. J. Immunol. 176, 6262–6269 (2006).
Nakae, S., Nambu, A., Sudo, K. & Iwakura, Y. Suppression of immune induction of collagen-induced arthritis in Il-17 deficient mice. J. Immunol. 171, 6173–6177 (2003).
Bush, K. A., Farmer, K. M., Walker, J. S. & Kirkham, B. W. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with IL-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 46, 802–805 (2002).
Lubberts, E. et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen induced arthritis reduces joint inflammation, cartilage destruction and bone erosion. Arthritis Rheum. 50, 650–659 (2004).
Koenders, M. I. et al. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am. J. Pathol. 167, 141–149 (2005).
Abdollahi-Roodsaz, S. et al. Shift from Toll-like receptor 2 (TLR2) toward TLR-4 dependency in the erosive stage of chronic streptococcal cell wall arthritis coincident with TLR-4-mediated interleukin-17 production. Arthritis Rheum. 58, 3753–3764 (2008).
Ogura, H. et al. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 29, 628–636 (2008).
Iwakura, Y. & Ishigame, H. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 116, 1218–1222 (2006).
Hirota, K. et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ TH cells that cause autoimmune arthritis. J. Exp. Med. 204, 41–47 (2007).
Nakae, S. et al. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl Acad. Sci. USA 100, 5986–5990 (2003).
Koenders, M. I. et al. IL-1 drives pathogenic TH17 cells during spontaneous arthritis in interleukin-1 receptor antagonist-deficient mice. Arthritis Rheum. 58, 3461–3470 (2008).
Abdollahi-Roodsaz, S. et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 118, 205–216 (2008).
Abdollahi-Roodsaz, S. et al. Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 56, 2957–2967 (2007).
Chabaud, M. et al. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 42, 963–970 (1999).
Chabaud, M. & Miossec, P. The combination of tumor necrosis factor-α blockade with interleukin-1 and interleukin-17 blockade is more effective for controlling synovial inflammation and bone resorption in an ex vivo model. Arthritis Rheum. 44, 1293–1303 (2001).
Chabaud, M., Lubberts, E., Joosten, L., van den Berg, W. B. & Miossec, P. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis Res. 3, 168–177 (2001).
Andersson, M. K. et al. Effects on osteoclast and osteoblast activities in cultured mouse calvarial bones by synovial fluids from patients with a loose joint prothesis and from osteoarthritis patients. Arthritis Res. Ther. 9, R18 (2007).
Annunziato, F. et al. Phenotypic and functional features of human TH17 cells. J. Exp. Med. 204, 1849–1861 (2007).
Wei, G. et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 (2009).
Lee, Y. K. et al. Late developmental plasticity in the T helper 17 lineage. Immunity 30, 92–107 (2009).
Martin-Orozco, N., Chung, Y., Chang, S. H., Wang, Y. H. & Dong, C. TH17 cells promote pancreatic inflammation but only induces diabetes efficiently in lymphopenic hosts after conversion into TH1 cells. Eur. J. Immunol. 39, 216–224 (2009).
Bending, D. et al. Highly purified TH17 cells from BDC2.5NOD mice convert into TH1-like cells in NOD/SCID recipient mice. J. Clin. Invest. 119, 565–572 (2009).
Kirkham, B. W. et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort). Arthritis Rheum. 54, 1122–1131 (2006).
Raza, K. et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal origin. Arthritis Res. Ther. 7, R784–R795 (2005).
Yamada, H. et al. TH1 but not TH17 cells predominate in the joints of patients with rheumatoid arthritis. Ann. Rheum. Dis. 67, 1299–1304 (2008).
Pène, J. et al. Chronically inflamed human tissues are infiltrated by highly differentiated TH17 lymphocytes. J. Immunol. 180, 7423–7430 (2008).
Nishala, K. et al. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 58, 875–887 (2008).
Shahrara, S., Huang, Q. Q., Mandelin, A. M. & Pope, R. M. TH17 cells in rheumatoid arthritis. Arthritis Res. Ther. 10, R93 (2008).
Evans, H. G. et al. In vivo activated monocytes from the site of inflammation in humans specifically promote TH17 responses. Proc. Natl Acad. Sci. USA 106, 6232–6237 (2009).
Egan, P. J., van Nieuwenhuijzen, A., Campbell, I. K. & Wicks, I. P. Promotion of the local differentiation of murine TH17 cells by synovial macrophages during acute inflammatory arthritis. Arthritis Rheum. 58, 3720–3729 (2008).
Peng, M. Y. et al. Interleukin-17 producing γδ T cells increased in patients with active pulmonary tuberculosis. Cell. Mol. Immunol. 5, 203–208 (2008).
Roark, C. L., Simonian, P. L., Fontenot, A. P., Born, W. K. & O'Brien, R. L. γδ T cells: an important source of IL-17. Curr. Opin. Immunol. 20, 353–357 (2008).
Michel, M. L. et al. Critical role of ROR-γt in a new thymic pathway leading to IL-17 producing invariant NKT cell differentiation. Proc. Natl Acad. Sci. USA 105, 19845–19850 (2008).
Takahashi, N. et al. IL-17 produced by Paneth cells drives TNF-induced shock. J. Exp. Med. 8, 1755–1761 (2008).
Ishigame, H. et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30, 108–119 (2009).
Milner, J. D. et al. Impaired TH17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452, 773–776 (2008).
Luger, D. et al. Either a TH17 or a TH1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J. Exp. Med. 205, 1517–1522 (2008).
Doodes, P. D. et al. Development of proteoglycan-induced arthritis is independent of IL-17. J. Immunol. 181, 329–337 (2008).
Van den Berg, W. B., van Lent, P. L., Joosten, L. A., Abdollahi-Roodsaz, S. & Koenders, M. I. Amplifying elements of arthritis and joint destruction. Ann. Rheum. Dis. 66 (Suppl. 3), iii45–iii48 (2007).
Ivanov, S. & Linden, A. Interleukin-17 as drug target in human disease. Trends Pharmacol. Sci. 30, 95–103 (2009).
Miossec, P. Diseases that may benefit from manipulating the TH17 pathway. Eur. J. Immunol. 39, 667–669 (2009).
Durez, P. et al. AIN457, an anti-IL-17 antibody, shows good safety and induces clinical responses in patients with active rheumatoid arthritis (RA) despite methotrexate therapy in a randomized, double-blind, proof-of-concept trial [abstract LB0002]. Ann. Rheum. Dis. 68 (Suppl. 3), 125 (2009).
Sloan-Lancaster, J., Genovese, M. C., Roberson, S. A. & van den Bosch, F. Safety, tolerability and evidence of efficacy of intravenous LY2439821 in patients with rheumatoid arthritis receiving background oral DMARDs [abstract OP-0160]. Ann. Rheum. Dis. 68 (Suppl. 3), 123 (2009).
Firestein, G. S. Evolving concepts of rheumatoid arthritis. Nature 423, 356–361 (2003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
van den Berg, W., Miossec, P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol 5, 549–553 (2009). https://doi.org/10.1038/nrrheum.2009.179
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2009.179
This article is cited by
-
Inhibitory effects of Acanthopanax sessiliflorus Harms extract on the etiology of rheumatoid arthritis in a collagen-induced arthritis mouse model
Arthritis Research & Therapy (2024)
-
Effects of targeted therapies on bone in rheumatic and musculoskeletal diseases
Nature Reviews Rheumatology (2022)
-
The Interrelationship Between Diabetes, IL-17 and Bone Loss
Current Osteoporosis Reports (2020)
-
Mechanisms of lung disease development in rheumatoid arthritis
Nature Reviews Rheumatology (2019)
-
Baicalin attenuates collagen-induced arthritis via inhibition of JAK2-STAT3 signaling and regulation of Th17 cells in mice
Journal of Cell Communication and Signaling (2019)