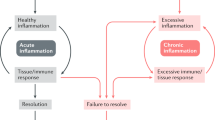
Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory synovitis that is dominated by the presence of macrophages, lymphocytes and synovial fibroblasts, which leads to the destruction of bone and cartilage. The effectiveness of therapies that are directed against tumour-necrosis factor and interleukin-1 has identified macrophages as a crucial target for therapeutic intervention. However, not all patients respond to these therapies, and the benefits of this form of treatment are short lived. Recent work indicates that the insufficient apoptosis of inflammatory cells in the RA joint might contribute to pathogenesis. In this article, I characterize the mechanisms that prevent the apoptosis of chronic inflammatory cells in the RA joint, to identify potential new targets for the treatment of RA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pope, R. M. & Perlman, H. Current Molecular Medicine: Principles of Molecular Rheumatology (ed. Tsokos, G. C.) 325–361 (Humana Press, Totowa, New Jersey, 2000).
Bathon, J. M. et al. Comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N. Engl. J. Med. 343, 1586–1593 (2000).
Feldmann, M. Pathogenesis of arthritis: recent research progress. Nature Immunol. 2, 771–773 (2001).
Feldmann, M. & Maini, R. N. Anti-TNFα therapy of rheumatoid arthritis: what have we learned? Annu. Rev. Immunol. 19, 163–196 (2001).
Lipsky, P. E. et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N. Engl. J. Med. 343, 1594–1602 (2000).
Sugiyama, M. et al. Localization of apoptosis and expression of apoptosis-related proteins in the synovium of patients with rheumatoid arthritis. Ann. Rheum. Dis. 55, 442–449 (1995).
Nakajima, T. et al. Apoptosis and functional Fas antigen in rheumatoid arthritis synoviocytes. Arthritis Rheum. 38, 485–491 (1995).
Matsumoto, S., Muller-Ladner, U., Gay, R. E., Nishioka, K. & Gay, S. Ultrastructural demonstration of apoptosis, Fas and Bcl-2 expression of rheumatoid synovial fibroblasts. J. Rheumatol. 23, 1345–1352 (1996).
Firestein, G. S. Apoptosis in rheumatoid arthritis synovium. J. Clin. Invest. 96, 1631–1638 (1995).
Fraser, A., Fearon, U., Reece, R., Emery, P. & Veale, D. J. Matrix metalloproteinase 9, apoptosis and vascular morphology in early arthritis. Arthritis Rheum. 44, 2024–2028 (2001).
Firestein, G. S. et al. Apoptosis in rheumatoid arthritis: p53 overexpression in rheumatoid arthritis synovium. Am. J. Pathol. 149, 2143–2151 (1996).
Perlman, H. et al. Differential expression pattern of the anti-apoptotic proteins, Bcl-2 and Flip in experimental arthritis. Arthritis Rheum. 44, 2899–2908 (2001).
Tak, P. P., Zvaifler, N. J., Green, D. R. & Firestein, G. S. Rheumatoid arthritis and p53: how oxidative stress might alter the course of inflammatory diseases. Immunol. Today 21, 78–82 (2000).
Lawrence, T., Gilroy, D. W., Colville-Nash, P. R. & Willoughby, D. A. Possible new role for NF-κB in the resolution of inflammation. Nature Med. 7, 1291–1297 (2001).
Savill, J. Apoptosis in resolution of inflammation. J. Leukocyte Biol. 61, 375–380 (1997).
Fadok, V. A., Bratton, D. L. & Henson, P. M. Phagocyte receptors for apoptotic cells: recognition, uptake and consequences. J. Clin. Invest. 108, 957–962 (2001).
van Lent, P. L. et al. Uptake of apoptotic leukocytes by synovial lining macrophages inhibits immune-complex-mediated arthritis. J. Leukocyte Biol. 70, 708–714 (2001).
Perlman, H. et al. Flice-inhibitory protein expression during macrophage differentiation confers resistance to Fas-mediated apoptosis. J. Exp. Med. 190, 1679–1688 (1999).
Perlman, H. et al. Rheumatoid arthritis synovial macrophages express the Fas-associated death domain-like interleukin-1β-converting enzyme-inhibitory protein and are refractory to Fas-mediated apoptosis. Arthritis Rheum. 44, 21–30 (2001).
Yeh, W.-C. et al. Requirement for casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity 12, 633–642 (2000).
Hohlbaum, A. M., Gregory, M. S., Ju, S. T. & Marshak-Rothstein, A. Fas ligand engagement of resident peritoneal macrophages in vivo induces apoptosis and the production of neutrophil chemotactic factors. J. Immunol. 167, 6217–6224 (2001).
Pagliari, L. J., Perlman, H., Liu, H. & Pope, R. M. Macrophages require constitutive NF-κB activation to maintain A1 expression and mitochondrial homeostasis. Mol. Cell. Biol. 20, 8855–8865 (2000).
Liu, H. et al. TNF-α gene expression in macrophages: regulation by NF-κB is independent of c-Jun or C/EBPβ. J. Immunol. 164, 4277–4285 (2000).
Liu, H., Perlman, H., Pagliari, L. & Pope, R. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages: role of Mcl-1, independent of NF-κB, Bad or caspase activation. J. Exp. Med. 194, 113–125 (2001).
Handel, M. L., McMorrow, L. B. & Gravallese, E. M. Nuclear factor-κB in rheumatoid synovium. Arthritis Rheum. 38, 1762–1770 (1995).
Davis, R. E., Brown, K. D., Siebenlist, U. & Staudt, L. M. Constitutive nuclear factor-κB activity is required for survival of active B-cell-like diffuse large B-cell lymphoma cells. J. Exp. Med. 194, 1861–1874 (2001).
Georganas, C. et al. Regulation of IL-6 and IL-8 expression in rheumatoid arthritis synovial fibroblasts: the dominant role for NF-κB but not C/EBPβ or c-Jun. J. Immunol. 165, 7199–7206 (2000).
Foxwell, B. et al. Efficient adenoviral infection with IκBα reveals that macrophage tumor necrosis factor-α production in rheumatoid arthritis is NF-κB dependent. Proc. Natl Acad. Sci. USA 95, 8211–8215 (1998).
Bondeson, J., Browne, K. A., Brennan, F. M., Foxwell, B. M. J. & Feldmann, M. Selective regulation of cytokine induction by adenoviral gene transfer of IκBα into human macrophages: lipopolysaccharide-induced, but not zymosan-induced, proinflammatory cytokines are inhibited, but IL-10 is nuclear factor-κB independent. J. Immunol. 162, 2939–2945 (1999).
Kawakami, A. et al. Inhibition of Fas-antigen-mediated apoptosis of rheumatoid synovial cells in vitro by transforming growth factor-β1. Arthritis Rheum. 39, 1267–1276 (1996).
Kobayashi, T. et al. Differential regulation of Fas-mediated apoptosis of rheumatoid synoviocytes by tumor necrosis factor-κ and basic fibroblast growth factor is associated with the expression of apoptosis-related molecules. Arthritis Rheum. 43, 1106–1114 (2000).
Franz, J. K. et al. Expression of sentrin, a novel antiapoptotic molecule, at sites of synovial invasion in rheumatoid arthritis. Arthritis Rheum. 43, 599–607 (2000).
Kobayashi, T. et al. Tumor necrosis factor-α regulation of the FAS-mediated apoptosis-signaling pathway in synovial cells. Arthritis Rheum. 42, 519–526 (1999).
Zhang, H.-G. et al. Gene therapy that inhibits nuclear translocation of nuclear factor-κB results in tumor necrosis factor-α-induced apoptosis of human synovial fibroblasts. Arthritis Rheum. 43, 1094–1105 (2000).
De Smaele, E. et al. Induction of gadd45β by NF-κB downregulates pro-apoptotic JNK signalling. Nature 414, 308–313 (2001).
Tang, G. et al. Inhibition of JNK activation through NF-κB target genes. Nature 414, 313–317 (2001).
Zhang, H. G. et al. Regulation of tumor necrosis factor-α-mediated apoptosis in rheumatoid arthritis synovial fibroblasts by the protein kinase Akt. Arthritis Rheum. 44, 1555–1567 (2001).
Pap, T. et al. Activation of synovial fibroblasts in rheumatoid arthritis: lack of expression of the tumour suppressor PTEN at sites of invasive growth and destruction. Arthritis Res. 2, 59–64 (2000).
Tak, P. P. et al. p53 overexpression in synovial tissue from patients with early and longstanding rheumatoid arthritis compared with patients with reactive arthritis and osteoarthritis. Arthritis Rheum. 42, 948–953 (1999).
Firestein, G. S., Echeverri, F., Yeo, M., Zvaifler, N. J. & Green, D. R. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc. Natl Acad. Sci. USA 94, 10895–10900 (1997).
Aupperle, K. R. et al. Regulation of synoviocyte proliferation, apoptosis and invasion by the p53 tumor suppressor gene. Am. J. Pathol. 152, 1091–1098 (1998).
Han, Z., Boyle, D. L., Shi, Y., Green, D. R. & Firestein, G. S. Dominant-negative p53 mutations in rheumatoid arthritis. Arthritis Rheum. 42, 1088–1092 (1999).
Pap, T., Aupperle, K. R., Gay, S., Firestein, G. S. & Gay, R. E. Invasiveness of synovial fibroblasts is regulated by p53 in the SCID mouse in vivo model of cartilage invasion. Arthritis Rheum. 44, 676–681 (2001).
Yamanishi, Y. et al. Regulation of joint destruction and inflammation by p53 in collagen-induced arthritis. Am. J. Pathol. 169, 123–130 (2002).
Smolen, J. S. & Steiner, G. Rheumatoid arthritis is more than cytokines: autoimmunity and rheumatoid arthritis. Arthritis Rheum. 44, 2218–2220 (2001).
Salmon, M. et al. Inhibition of T-cell apoptosis in the rheumatoid synovium. J. Clin. Invest. 99, 439–446 (1997).
Cantwell, M. J., Hua, T., Zvaifler, N. J. & Kipps, T. J. Deficient Fas ligand expression by synovial lymphocytes from patients with rheumatoid arthritis. Arthritis Rheum. 40, 1644–1652 (1997).
Kolenko, V. et al. Inhibition of NF-κB activity in human T lymphocytes induces caspase-dependent apoptosis without detectable activation of caspase-1 and -3. J. Immunol. 163, 590–598 (1999).
Moreland, L. W. et al. Double-blind, placebo-controlled multicenter trial using chimeric monoclonal anti-CD4 antibody, cM-T412, in rheumatoid arthritis patients receiving concomitant methotrexate. Arthritis Rheum. 38, 1581–1588 (1995).
Ito, M. R. et al. Rheumatic diseases in an MRL strain of mice with a deficit in the functional Fas ligand. Arthritis Rheum. 40, 1054–1063 (1997).
Hsu, H. C. et al. Defective Fas-ligand-mediated apoptosis predisposes to development of a chronic erosive arthritis subsequent to Mycoplasma pulmonis infection. Arthritis Rheum. 44, 2146–2159 (2001).
Zhang, J., Bardos, T., Mikecz, K., Finnegan, A. & Glant, T. T. Impaired Fas signalling pathway is involved in defective T-cell apoptosis in autoimmune murine arthritis. J. Immunol. 166, 4981–4986 (2001).
Zhou, T. et al. Bisindolylmaleimide VIII facilitates Fas-mediated apoptosis and inhibits T-cell-mediated autoimmune diseases. Nature Med. 5, 42–48 (1999).
Harjacek, M. et al. Expression of galectins-1 and -3 correlates with defective mononuclear-cell apoptosis in patients with juvenile idiopathic arthritis. J. Rheumatol. 28, 1914–1922 (2001).
Rabinovich, G. A. et al. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T-cell apoptosis. J. Exp. Med. 190, 385–398 (1999).
Zhang, H. et al. Amelioration of collagen-induced arthritis by CD95 (Apo-1/Fas)-ligand gene transfer. J. Clin. Invest. 100, 1951–1957 (1997).
Yao, Q. et al. Adenoviral-mediated delivery of FAS ligand to arthritic joints causes extensive apoptosis in the synovial lining. J. Gene Med. 2, 210–219 (2000).
Sakai, K. et al. Potential withdrawal of rheumatoid synovium by the induction of apoptosis using a novel in vivo model of rheumatoid arthritis. Arthritis Rheum. 41, 1251–1257 (1998).
Okamoto, K. et al. Induction of apoptosis in the rheumatoid synovium by Fas ligand gene transfer. Gene Ther. 5, 331–338 (1998).
Tomita, T. et al. Suppressed severity of collagen-induced arthritis by in vivo transfection of nuclear factor-κB decoy oligodeoxynucleotides as a gene therapy. Arthritis Rheum. 42, 2532–2542 (1999).
Tak, P. P. et al. Inhibitor of nuclear factor-κB kinase-β is a key regulator of synovial inflammation. Arthritis Rheum. 44, 1897–1907 (2001).
Miagkov, A. V. et al. NF-κB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc. Natl Acad. Sci. USA 95, 13859–13864 (1998).
Yao, Q. et al. Gene transfer of p53 to arthritic joints stimulates synovial apoptosis and inhibits inflammation. Mol. Ther. 3, 901–910 (2001).
Gerlag, D. M. et al. Suppression of murine collagen-induced arthritis by targeted apoptosis of synovial neovasculature. Arthritis Res. 3, 357–361 (2001).
Kong, Y. Y., Boyle, W. J. & Penninger, J. M. Osteoprotegerin ligand: a regulator of immune responses and bone physiology. Immunol. Today 21, 495–502 (2000).
Faouzi, S. et al. Anti-Fas induces hepatic chemokines and promotes inflammation by an NF-κB-independent, caspase-3-dependent pathway. J. Biol. Chem. 276, 49077–49082 (2001).
Fujii, K., Fujii, Y., Hubscher, S. & Tanaka, Y. CD44 is the physiological trigger of Fas up-regulation on rheumatoid synovial cells. J. Immunol. 167, 1198–1203 (2001).
Cutolo, M., Sulli, A., Pizzorni, C., Seriolo, B. & Straub, R. H. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann. Rheum. Dis. 60, 729–735 (2001).
Nakazawa, F. et al. Methotrexate inhibits rheumatoid synovitis by inducing apoptosis. J. Rheumatol. 28, 1800–1808 (2001).
Cutolo, M. et al. Antiproliferative and antiinflammatory effects of methotrexate on cultured differentiating myeloid monocytic cells (THP-1) but not on synovial macrophages from patients with rheumatoid arthritis. J. Rheumatol. 27, 2551–2557 (2000).
Genestier, L. et al. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J. Clin. Invest. 102, 322–328 (1998).
Rodenburg, R. J., Ganga, A., van Lent, P. L., van de Putte, L. B. & van Venrooij, W. J. The antiinflammatory drug sulfasalazine inhibits tumor necrosis factor-α expression in macrophages by inducing apoptosis. Arthritis Rheum. 43, 1941–1950 (2000).
Lugering, A. et al. Infliximab induces apoptosis in monocytes from patients with chronic active Crohn's disease by using a caspase-dependent pathway. Gastroenterology 121, 1145–1157 (2001).
Frith, J. C., Monkkonen, J., Auriola, S., Monkkonen, H. & Rogers, M. J. The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum. 44, 2201–2210 (2001).
Barrera, P. et al. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 43, 1951–1959 (2000).
Gratiot-Deans, J., Ding, L., Turka, L. A. & Nunez, G. Bcl-2 proto-oncogene expression during human T-cell development. Evidence for biphasic regulation. J. Immunol. 151, 83–91 (1993).
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Entrez
LocusLink
Medscape DrugInfo
OMIM
Rights and permissions
About this article
Cite this article
Pope, R. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat Rev Immunol 2, 527–535 (2002). https://doi.org/10.1038/nri846
Issue Date:
DOI: https://doi.org/10.1038/nri846
This article is cited by
-
Role of serum survivin as a predictor of response to biological treatment in rheumatoid arthritis patients
Egyptian Rheumatology and Rehabilitation (2023)
-
Ceria-vesicle nanohybrid therapeutic for modulation of innate and adaptive immunity in a collagen-induced arthritis model
Nature Nanotechnology (2023)
-
Fluorinated polyamidoamine dendrimer-mediated miR-23b delivery for the treatment of experimental rheumatoid arthritis in rats
Nature Communications (2023)
-
A molecular insight of inflammatory cascades in rheumatoid arthritis and anti-arthritic potential of phytoconstituents
Molecular Biology Reports (2022)
-
Shikonin attenuates rheumatoid arthritis by targeting SOCS1/JAK/STAT signaling pathway of fibroblast like synoviocytes
Chinese Medicine (2021)