Key Points
-
Regulatory T cells represent a distinct T-cell subset that has a key role in inducing and maintaining immunological tolerance. Several different regulatory T-cell subsets have been described. This Review focuses on forkhead box P3 (FOXP3)+CD4+CD25+ T cells, being the only natural occurring regulatory T-cell subset described so far, and on T regulatory type 1 cells, being the only inducible subset used in the clinic to date.
-
Compelling data generated in preclinical animal models indicate that regulatory T cells can be used as therapeutic agents for inducing tolerance to alloantigens after transplantation or for re-establishing self-tolerance in autoimmune diseases.
-
The possibility that human regulatory T cells might have an application for the treatment of T-cell-mediated diseases has recently gained increasing momentum. However, it is still unclear: which regulatory T-cell subset is most appropriate for adoptive transfer; which method should be used for their ex vivo expansion or differentiation; which human disease would benefit the most from regulatory T-cell transfer; and whether a combined therapy with other drugs will be needed.
-
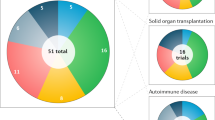
At present, a few clinical trials of human regulatory T-cell-based immunotherapy are ongoing after bone-marrow transplantation. These trials will hopefully pave the way for the application of this therapy to other immune-mediated diseases.
-
The common future clinical application of regulatory T-cell-based immunotherapy strongly depends on safety, ethical and economical issues. This therapeutic approach must be safe and prove to be of superior efficacy over conventional therapy. Furthermore, in its initial experimental phase, regulatory T-cell-based immunotherapy is extremely expensive and requires good manufacturing practice certified facilities.
-
Should this immunotherapy meet its therapeutic target, one could envisage that not only academic institutions but also pharmaceutical companies will be interested in adopting this therapeutic approach.
Abstract
Substantial progress in understanding the biology of regulatory T cells and their roles in health and disease has been achieved in the past 10 years. This has led to an increasing interest in the possibility of using regulatory T cells as a biological therapy to preserve and restore tolerance to self antigens and alloantigens. Immunotherapy by the adoptive transfer of regulatory T cells may have several advantages over conventional treatments. However, several hurdles have to be overcome before such a therapy can enter clinical practice. This Review summarizes our current knowledge of regulatory T cells, illustrates the ongoing regulatory T-cell-based clinical trials, analyses the strengths and pitfalls of this new therapeutic approach, and highlights the future perspectives.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Shevach, E. M. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity 25, 195–201 (2006).
Groux, H. et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389, 737–742 (1997). In this paper, both human and mouse IL-10-producing T R 1 cells were characterized for the first time.
Walker, M. R. et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25− T cells. J. Clin. Invest. 112, 1437–1443 (2003).
Sakaguchi, S., Setoguchi, R., Yagi, H. & Nomura, T. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in self-tolerance and autoimmune disease. Curr. Top. Microbiol. Immunol. 305, 51–66 (2006).
Taams, L. S. et al. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur. J. Immunol. 32, 1621–1630 (2002).
Wing, K. et al. CD4 T cell activation by myelin oligodendrocyte glycoprotein is suppressed by adult but not cord blood CD25+ T cells. Eur. J. Immunol. 33, 579–587 (2003).
Danke, N. A., Koelle, D. M., Yee, C., Beheray, S. & Kwok, W. W. Autoreactive T cells in healthy individuals. J. Immunol. 172, 5967–5972 (2004).
Kitani, A., Chua, K., Nakamura, K. & Strober, W. Activated self-MHC-reactive T cells have the cytokine phenotype of Th3/T regulatory cell 1 T cells. J. Immunol. 165, 691–702 (2000).
Arif, S. et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J. Clin. Invest. 113, 451–463 (2004). This is the first study providing evidence that patients with type 1 diabetes have reduced numbers of circulating islet-specific T R 1 cells compared with HLA-matched healthy controls.
Baecher-Allan, C. & Hafler, D. A. Human regulatory T cells and their role in autoimmune disease. Immunol. Rev. 212, 203–216 (2006).
Yudoh, K., Matsuno, H., Nakazawa, F., Yonezawa, T. & Kimura, T. Reduced expression of the regulatory CD4+ T cell subset is related to Th1/Th2 balance and disease severity in rheumatoid arthritis. Arthritis Rheum. 43, 617–627 (2000).
Miura, Y. et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood 104, 2187–2193 (2004). This study shows for the first time a significant reduction of FOXP3 mRNA levels in PBMCs from patients with GVHD compared with those without GVHD.
Rezvani, K. et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood 108, 1291–1297 (2006).
Zorn, E. et al. Reduced frequency of FOXP3+CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood 106, 2903–2911 (2005).
Clark, F. J. et al. Chronic graft-versus-host disease is associated with increased numbers of peripheral blood CD4+CD25high regulatory T cells. Blood 103, 2410–2416 (2004).
Meignin, V. et al. Numbers of Foxp3-expressing CD4+CD25high T cells do not correlate with the establishment of long-term tolerance after allogeneic stem cell transplantation. Exp. Hematol. 33, 894–900 (2005).
Rieger, K. et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood 107, 1717–1723 (2006).
Meloni, F. et al. Monocyte chemoattractant protein-1 levels in bronchoalveolar lavage fluid of lung-transplanted patients treated with tacrolimus as rescue treatment for refractory acute rejection. Transplant Proc. 35, 1523–1526 (2003).
Demirkiran, A. et al. Low circulating regulatory T-cell levels after acute rejection in liver transplantation. Liver Transpl. 12, 277–284 (2006).
Salama, A. D., Najafian, N., Clarkson, M. R., Harmon, W. E. & Sayegh, M. H. Regulatory CD25+ T cells in human kidney transplant recipients. J. Am. Soc. Nephrol. 14, 1643–1651 (2003).
Bacchetta, R. et al. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J. Exp. Med. 179, 493–502 (1994). This study describes the successful isolation of CD4+ host-reactive T-cell clones from SCID patients transplanted with allogeneic HSCs, which produce high amounts of IL-10 in the absence of IL-4 after antigen-specific stimulation in vitro . The presence of these IL-10-producing CD4+ T cells correlated with the absence of GVHD and long-term tolerance.
Baker, K. et al. High spontaneous IL-10 production in unrelated bone marrow transplant recipients is associated with fewer transplant-related complications and early deaths. Bone Marrow Transplant. 23, 1123–1129 (1999).
Weston, L. E., Geczy, A. F. & Briscoe, H. Production of IL-10 by alloreactive sibling donor cells and its influence on the development of acute GVHD. Bone Marrow Transplant. 37, 207–212 (2006).
VanBuskirk, A. M. et al. Human allograft acceptance is associated with immune regulation. J. Clin. Invest. 106, 145–155 (2000).
Bacchetta, R. et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J. Clin. Invest. 116, 1713–1722 (2006).
Marangoni, F. et al. WASP regulates suppressor activity of human and murine CD4+CD25+FOXP3+ natural regulatory T cells. J. Exp. Med. 204, 369–380 (2007).
Kriegel, M. A. et al. Defective suppressor function of human CD4+ CD25+ regulatory T cells in autoimmune polyglandular syndrome type II. J. Exp. Med. 199, 1285–1291 (2004).
Bleesing, J. J. et al. Immunophenotypic profiles in families with autoimmune lymphoproliferative syndrome. Blood 98, 2466–2473 (2001).
Mottet, C., Uhlig, H. H. & Powrie, F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 170, 3939–3943 (2003).
Tang, Q. et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 199, 1455–1465 (2004).
Tarbell, K. V. et al. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J. Exp. Med. 204, 191–201 (2007).
Hanash, A. M. & Levy, R. B. Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood 105, 1828–1836 (2005).
Joffre, O., Gorsse, N., Romagnoli, P., Hudrisier, D. & van Meerwijk, J. P. Induction of antigen-specific tolerance to bone marrow allografts with CD4+CD25+ T lymphocytes. Blood 103, 4216–4221 (2004).
Taylor, P. A. et al. L-selectinhi but not the L-selectinlo CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood 104, 3804–3812 (2004).
Cohen, J. L., Trenado, A., Vasey, D., Klatzmann, D. & Salomon, B. L. CD4+CD25+ immunoregulatory T cells: new therapeutics for graft-versus-host disease. J. Exp. Med. 196, 401–406 (2002).
Hoffmann, P., Ermann, J., Edinger, M., Fathman, C. G. & Strober, S. Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Exp. Med. 196, 389–399 (2002). References 35, 36 and 96 are the first to show that the adoptive transfer of CD4+CD25+ regulatory T cells significantly delays or even prevents GVHD in preclinical mouse models of BMT.
Trenado, A. et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J. Clin. Invest. 112, 1688–1696 (2003).
Zeller, J. C. et al. Induction of CD4+ T cell alloantigen-specific hyporesponsiveness by IL-10 and TGF-β. J. Immunol. 163, 3684–3691 (1999).
Gregori, S. et al. Regulatory T cells induced by 1α,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J. Immunol. 167, 1945–1953 (2001).
Lee, M. K. 4th. et al. Promotion of allograft survival by CD4+CD25+ regulatory T cells: evidence for in vivo inhibition of effector cell proliferation. J. Immunol. 172, 6539–6544 (2004).
Battaglia, M., Stabilini, A. & Roncarolo, M. G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105, 4743–4748 (2005). In this study, we showed, for the first time, that rapamycin has the ability to allow the selective proliferation of T Reg cells.
Battaglia, M. et al. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes 55, 40–49 (2006).
Waldmann, H., Adams, E., Fairchild, P. & Cobbold, S. Infectious tolerance and the long-term acceptance of transplanted tissue. Immunol. Rev. 212, 301–313 (2006).
Barrat, F. J. et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 195, 603–616 (2002).
Hoffmann, P. et al. Isolation of CD4+CD25+ regulatory T cells for clinical trials. Biol. Blood Marrow Transplant. 12, 267–274 (2006).
Hoffmann, P., Eder, R., Kunz-Schughart, L. A., Andreesen, R. & Edinger, M. Large-scale in vitro expansion of polyclonal human CD4+CD25high regulatory T cells. Blood 104, 895–903 (2004).
Levings, M. K. et al. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor β, but not interleukin 10, and are distinct from type 1 T regulatory cells. J. Exp. Med. 196, 1335–1346 (2002).
Battaglia, M. et al. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. 177, 8338–8347 (2006).
Liu, W. et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 203, 1701–1711 (2006).
Seddiki, N. et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203, 1693–1700 (2006).
Jiang, S., Camara, N., Lombardi, G. & Lechler, R. I. Induction of allopeptide-specific human CD4+CD25+ regulatory T cells ex vivo. Blood 102, 2180–2186 (2003). References 50 and 51 show that CD4+CD25+ T Reg cells are CD127low/− and open new venues for a better isolation and purification of T Reg cells.
Roncarolo, M. G. et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 212, 28–50 (2006).
Battaglia, M. & Roncarolo, M. G. Induction of transplantation tolerance via regulatory T cells. Inflamm. Allergy Drug Targets 5, 157–165 (2006).
Zeiser, R. et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood 108, 390–399 (2006).
Achenbach, P., Bonifacio, E. & Ziegler, A. G. Predicting type 1 diabetes. Curr. Diab. Rep. 5, 98–103 (2005).
Ermann, J. et al. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood 105, 2220–2226 (2005).
Nguyen, V. H. et al. In vivo dynamics of regulatory T cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood 109, 2649–2656 (2007).
Misra, N., Bayry, J., Lacroix-Desmazes, S., Kazatchkine, M. D. & Kaveri, S. V. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J. Immunol. 172, 4676–4680 (2004).
Venet, F. et al. Human CD4+CD25+ regulatory T lymphocytes inhibit lipopolysaccharide-induced monocyte survival through a Fas/Fas ligand-dependent mechanism. J. Immunol. 177, 6540–6547 (2006).
Taams, L. S. et al. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum. Immunol. 66, 222–230 (2005).
Lewkowicz, P., Lewkowicz, N., Sasiak, A. & Tchorzewski, H. Lipopolysaccharide-activated CD4+CD25+ T regulatory cells inhibit neutrophil function and promote their apoptosis and death. J. Immunol. 177, 7155–7163 (2006).
Wood, K. J. & Sawitzki, B. Interferon γ: a crucial role in the function of induced regulatory T cells in vivo. Trends Immunol. 27, 183–187 (2006).
Farrar, M. A. & Schreiber, R. D. The molecular cell biology of interferon-γ and its receptor. Annu. Rev. Immunol. 11, 571–611 (1993).
Tang, Q. et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nature Immunol. 7, 83–92 (2006).
Sarween, N. et al. CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J. Immunol. 173, 2942–2951 (2004).
Chen, Z., Herman, A. E., Matos, M., Mathis, D. & Benoist, C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J. Exp. Med. 202, 1387–1397 (2005).
Roncarolo, M. G., Battaglia, M. & Gregori, S. The role of interleukin 10 in the control of autoimmunity. J. Autoimmun. 20, 269–272 (2003).
Peng, Y., Laouar, Y., Li, M. O., Green, E. A. & Flavell, R. A. TGF-β regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc. Natl Acad. Sci. USA 101, 4572–4577 (2004).
Battaglia, M. et al. Induction of tolerance in type 1 diabetes via both CD4+CD25+ T regulatory cells and T regulatory type 1 cells. Diabetes 55, 1571–1580 (2006).
Chen, C., Lee, W. H., Zhong, L. & Liu, C. P. Regulatory T cells can mediate their function through the stimulation of APCs to produce immunosuppressive nitric oxide. J. Immunol. 176, 3449–3460 (2006).
Battaglia, M., Gregori, S., Bacchetta, R. & Roncarolo, M. G. Tr1 cells: from discovery to their clinical application. Semin. Immunol. 18, 120–127 (2006).
Sakaguchi, S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nature Immunol. 6, 345–352 (2005).
Allan, S. E. et al. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J. Clin. Invest. 115, 3276–3284 (2005).
Singh, B. et al. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 182, 190–200 (2001).
Shevach, E. M. et al. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol. Rev. 212, 60–73 (2006).
Scheffold, A., Hühn, J. & Höfer, T. Regulation of CD4+CD25+ regulatory T cell activity: it takes (IL-) two to tango. Eur. J. Immunol. 35, 1336–1341 (2005).
Levings, M. K. et al. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood 105, 1162–1169 (2005).
Levings, M. K. et al. IFN-α and IL-10 induce the differentiation of human type 1 T regulatory cells. J. Immunol. 166, 5530–5539 (2001).
Ziegler, S. F. FOXP3: of mice and men. Annu. Rev. Immunol. 24, 209–226 (2006).
Khattri, R., Cox, T., Yasayko, S. A. & Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature Immunol. 4, 337–342 (2003).
Gondek, D. C., Lu, L. F., Quezada, S. A., Sakaguchi, S. & Noelle, R. J. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J. Immunol. 174, 1783–1786 (2005).
Zhao, D. M., Thornton, A. M., DiPaolo, R. J. & Shevach, E. M. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood 107, 3925–3932 (2006).
Grossman, W. J. et al. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 21, 589–601 (2004).
Chen, D. et al. CD4+CD25+ regulatory T-cells inhibit the islet innate immune response and promote islet engraftment. Diabetes 55, 1011–1021 (2006).
Ding, Q. et al. B7H1-Ig fusion protein activates the CD4+ IFN-γ receptor+ type 1 T regulatory subset through IFN-γ-secreting Th1 cells. J. Immunol. 177, 3606–3614 (2006).
Tarbell, K. V., Yamazaki, S., Olson, K., Toy, P. & Steinman, R. M. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J. Exp. Med. 199, 1467–1477 (2004).
Masteller, E. L. et al. Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. J. Immunol. 175, 3053–3059 (2005).
Chen, C., Lee, W. H., Yun, P., Snow, P. & Liu, C. P. Induction of autoantigen-specific Th2 and Tr1 regulatory T cells and modulation of autoimmune diabetes. J. Immunol. 171, 733–744 (2003).
You, S. et al. Presence of diabetes-inhibiting, glutamic acid decarboxylase-specific, IL-10-dependent, regulatory T cells in naive nonobese diabetic mice. J. Immunol. 173, 6777–6785 (2004).
Hori, S., Haury, M., Coutinho, A. & Demengeot, J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc. Natl Acad. Sci. USA 99, 8213–8218 (2002).
Kohm, A. P., Carpentier, P. A., Anger, H. A. & Miller, S. D. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 169, 4712–4716 (2002).
Morgan, M. E. et al. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 52, 2212–2221 (2005).
Cong, Y., Weaver, C. T., Lazenby, A. & Elson, C. O. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J. Immunol. 169, 6112–6119 (2002).
Scalapino, K. J., Tang, Q., Bluestone, J. A., Bonyhadi, M. L. & Daikh, D. I. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J. Immunol. 177, 1451–1459 (2006).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunol. 4, 330–336 (2003).
Taylor, P. A., Lees, C. J. & Blazar, B. R. The infusion of ex vivo activated and expanded CD4+CD25+ immune regulatory cells inhibits graft-versus-host disease lethality. Blood 99, 3493–3499 (2002).
Acknowledgements
The authors thank R. Bacchetta and S. Gregori (HSR-TIGET) for scientific discussions. M.-G.R. is supported by grants from the Telethon Foundation, RISET and the Juvenile Diabetes Research Foundation (JDRF). M.B. is supported by grants from Telethon and the JDRF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Negative selection
-
The deletion of self-reactive thymocytes in the thymus. Thymocytes expressing T-cell receptors that strongly recognize self peptide bound to self MHC molecules undergo apoptosis in response to the signalling generated by high-affinity binding.
- Graft-versus-host disease
-
(GVHD). A frequent complication after allogeneic stem-cell transplantation caused by the expansion of donor lymphocytes with helper and cytotoxic reactivity against host histocompatibility antigens. It can occur as two distinct syndromes: acute GVHD (occurring within 100 days) and chronic GVHD (occurring after 100 days). Complete depletion of T cells from the transplant largely eliminates GVHD but significantly increases the risk of graft failure and infections.
- Mixed chimerism
-
A state of coexistence of the host and allogeneic donor haematopoietic cells.
- IPEX
-
(Immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome). A disease caused by mutations in FOXP3 (forkhead box P3) and characterized by refractory enteritis and, in some patients, autoimmune endocrinopathies, autoimmune diabetes and thyroiditis. Unlike scurfy mice, peripheral-blood mononuclear cells from IPEX patients fail to produce cytokines after in vitro stimulation.
- WAS
-
(Wiskott–Aldrich syndrome). A life-threatening X-linked immunodeficiency caused by mutation in the WAS protein. It is characterized by thrombocytopaenia with small platelets, eczema, recurrent infections caused by immunodeficiency, and an increased incidence of autoimmune manifestations and malignancies.
- APS
-
(Autoimmune polyglandular syndrome; also known as APECED). APS type 1 is caused by the loss of central tolerance due to mutations in autoimmune regulator (AIRE), where as APS type 2 is of unknown pathogenesis besides an association with polymorphisms in CTLA4 and an HLA-extended haplotype. It is characterized by multiple endocrine diseases initiated by an autoimmune process.
- ALPS
-
(Autoimmune lymphoproliferative syndrome). A syndrome associated with diffuse autoimmune manifestations. ALPS type Ia patients have mutations in TNFRSF6, which encodes CD95; ALPS type Ib patients have mutations in TNFSF6, which encodes CD95 ligand; ALPS type II patients have mutations in CASP10, which encodes caspase-10.
- Homeostatic proliferation
-
Spontaneous proliferation of T cells in lymphopenic conditions, caused by chronic diseases or treatments such as thymectomy or irradiation. Factors that support T-cell homeostatic proliferation include peptide–MHC–TCR interactions and cytokines such as interleukin-7 and interleukin-15.
- Rapamycin
-
An immunosuppressive drug that, in contrast to calcineurin inhibitors (such as cyclosporin A and FK506), does not prevent T-cell activation but blocks interleukin-2-mediated clonal expansion by blocking mTOR (mammalian target of rapamycin). It does not interfere with the function and expansion of naturally occurring regulatory T cells.
- Bystander suppression
-
Suppression in which responses to a second, unrelated antigen can be inhibited when it is presented together with the antigen towards which tolerance has been already established.
- Leukapheresis
-
A laboratory procedure for separating high numbers of leukocytes from peripheral blood.
- Cytokine storm
-
A strong systemic immune response that results in the release of more than 150 inflammatory mediators (cytokines, oxygen free radicals and coagulation factors). Both pro-inflammatory cytokines (such as tumour-necrosis factor, interleukin-1 (IL-1) and IL-6) and anti-inflammatory cytokines (such as IL-10 and IL-1 receptor antagonist) are elevated in the serum of patients experiencing a cytokine storm.
- Scurfy mice
-
Loss-of-function mutations of Foxp3 in scurfy mice inhibit the development of naturally occurring regulatory T cells, resulting in a highly dysregulated immune system and consequent aggressive autoimmunity. These mice show hyperproduction of cytokines and increased numbers of memory T cells.
- CD95–CD95 ligand apoptotic pathway
-
CD95 ligand (also known as FAS ligand) binds to CD95 (FAS), which results in the formation of the death-inducing signalling complex and subsequent activation of caspases. Donor CD4+ T cells with killing capability preferentially use this pathway during acute graft-versus-host disease.
- Interleukin-1 receptor antagonist
-
(IL-1RA). A secreted protein that binds to IL-1R, thereby blocking IL-1R downstream signalling. IL-1RA inhibits the pro-inflammatory properties of IL-1α and IL-1β.
- Nitric oxide
-
(NO). A small molecule synthesized, mainly in macrophages, from arginine by nitric oxide synthase (NOS) enzymes. Increased levels of NO are found in inflammatory and autoimmune diseases, and during allograft rejection. It is the effector cytotoxic molecule responsible for macrophage-mediated cytotoxicity but can also suppress T-cell proliferation.
- Graft-versus-leukaemia
-
(GVL). An immune response mounted by the transplanted cells against the tumour cells of the host and it is one of the reasons that allogeneic transplants can be curative for cancer.
- Donor lymphocyte infusion
-
(DLI). Delayed administration of peripheral donor lymphocytes after T-cell-depleted bone-marrow transplantation in cancer patients. DLI plays a central role in both attacking the tumour cells and providing immune reconstitution. However, its use is limited by the risk of severe acute and chronic graft-versus-host disease.
Rights and permissions
About this article
Cite this article
Roncarolo, MG., Battaglia, M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol 7, 585–598 (2007). https://doi.org/10.1038/nri2138
Issue Date:
DOI: https://doi.org/10.1038/nri2138
This article is cited by
-
The role of dendritic cells in the immunomodulation to implanted biomaterials
International Journal of Oral Science (2022)
-
Immunosuppressive biomaterial-based therapeutic vaccine to treat multiple sclerosis via re-establishing immune tolerance
Nature Communications (2022)
-
IL-35 is critical in suppressing superantigenic Staphylococcus aureus-driven inflammatory Th17 responses in human nasopharynx-associated lymphoid tissue
Mucosal Immunology (2020)
-
Immune heterogeneity of head and tail pancreatic lymph nodes in non-obese diabetic mice
Scientific Reports (2019)
-
Lag3+ regulatory T lymphocytes in critical carotid artery stenosis
Clinical and Experimental Medicine (2019)