Key Points
-
The initiation and the progression of autoimmune diseases are controlled by complex interplay between cells of the innate immune system and the adaptive immune system.
-
Emerging evidence indicates that natural killer (NK) cells, which are components of the innate immune system, and autoreactive T cells, which are components of the adaptive immune system, undergo crosstalk during the initiation and the progression of autoimmunity.
-
Individuals with genetic defects in the number or the function of NK cells are predisposed to the development of autoimmune diseases, at least in some cases. However, studies of mice that have defects in NK-cell number or function, or mice that have been treated with NK-cell-depleting antibodies, have shown that NK cells can have either a beneficial or a deleterious influence on the generation of autoreactive T-cell responses, depending on the particular animal model that is used, the stage (initiation versus progression) of the disease that is studied and the experimental procedures that are used.
-
On the one hand, NK cells might promote the generation of autoreactive T cells by producing pro-inflammatory cytokines (such as interferon-γ), activating antigen-presenting cells, providing co-stimulatory signals to T cells and/or presenting antigens directly to T cells. On the other hand, NK cells might inhibit the generation of autoreactive T cells by producing regulatory cytokines (such as interleukin-10, IL-10), lysing antigen-presenting cells or T cells, or modulating the activity of other regulatory cells (such as natural killer T cells and CD4+CD25+ regulatory T cells).
-
Humans and mice with established autoimmunity have defects in the number and the function of NK cells, a phenomenon that is known as NK-cell degeneration.
-
Recent studies have provided evidence that autoreactive T cells might promote NK-cell degeneration, in a mechanism that probably involves IL-21 production by autoreactive T cells. As such, autoreactive T cells might 'paralyse' NK cells, perhaps to avoid the disease-promoting effects of NK cells.
-
To improve our understanding of the complex interactions between NK cells and autoreactive T cells, new immunological tools (such as antibodies that can selectively deplete NK cells) and genetic tools (such as gene-knockout mice that selectively lack NK cells) need to be developed.
-
A better understanding of the interactions between NK cells and autoreactive T cells might be exploited to develop therapies that target NK cells for patients with autoimmune disease.
Abstract
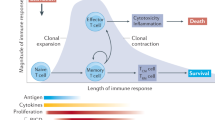
The initiation and the progression of autoimmune diseases stem from complex interactions that involve cells of both the innate and the adaptive immune system. As we discuss here, natural killer (NK) cells, which are components of the innate immune system, can inhibit or promote the activation of autoreactive T cells during the initiation of autoimmunity. After they have been activated, autoreactive T cells contribute to the homeostatic contraction of NK-cell populations. The dynamic interaction between NK cells and autoreactive T cells might indicate the transition from the innate immune triggering of autoimmunity to the progressive phase of the disease. Understanding the mechanisms and signals that control the reciprocal regulation of NK cells and autoreactive T cells could have important implications for treatment in the clinic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shi, F.-D., Ljunggren, H. G. & Sarvetnick, N. Innate immunity and autoimmunity: from self-protection to self-destruction. Trends Immunol. 22, 97–101 (2001).
McClain, M. T. et al. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nature Med. 11, 85–89 (2005).
Fearon, D. T. & Locksley, R. M. The instructive role of innate immunity in the acquired immune response. Science 272, 50–53 (1996).
Pulendran, B. & Ahmed, R. Translating innate immunity into immunological memory: implications for vaccine development. Cell 124, 849–863 (2006).
King, C., Ilic, A., Koelsch, K. & Sarvetnick, N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell 117, 265–277 (2004).
Yokoyama, W. M. & Plougastel, B. F. Immune functions encoded by the natural killer gene complex. Nature Rev. Immunol. 3, 304–316 (2003).
Lanier, L. L. Natural killer cell receptor signaling. Curr. Opin. Immunol. 15, 308–314 (2003).
Karre, K., Ljunggren, H. G., Piontek, G. & Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defense strategy. Nature 319, 675–678 (1986). This landmark paper shows that the amount of self MHC class I expression determines whether cells are targeted by NK cells, forming the basis of the 'missing-self' hypothesis.
Raulet, D. H. Interplay of natural killer cells and their receptors with the adaptive immune response. Nature Immunol. 5, 996–1002 (2004).
Yokoyama, W. M., Kim, S. & French, A. R. The dynamic life of natural killer cells. Annu. Rev. Immunol. 22, 405–429 (2004).
Lkawa, T., Kawamoto, H., Fujimoto, S. & Katsura, Y. Commitment of common T/natural killer (NK) progenitors to unipotent T and NK progenitors in the murine fetal thymus revealed by a single progenitor assay. J. Exp. Med. 190, 1617–1626 (1999).
Prlic, M., Blazar, B. R., Farrar, M. A. & Jameson, S. C. In vivo survival and homeostatic proliferation of natural killer cells. J. Exp. Med. 197, 967–976 (2003).
Kalberer, C. P., Siegler, U. & Wodnar-Filipowicz, A. Human NK cell development in NOD/SCID mice receiving grafts of cord blood CD34+ cells. Blood 102, 127–135 (2003).
Glimcher, L. H., Townsend, M. J., Sullivan, B. M. & Lord, G. M. Recent developments in the transcriptional regulation of cytolytic effector cells. Nature Rev. Immunol. 4, 900–911 (2004).
Jiang, K. et al. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nature Immunol. 1, 419–425 (2000).
Jiang, K. et al. Syk regulation of phosphoinositide 3-kinase-dependent NK cell function. J. Immunol. 168, 3155–3164 (2002).
Biron, C. A., Nguyen, K. B., Pien, G. C., Cousens, L. P. & Salazar-Mather, T. P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17, 189–220 (1999).
Peritt, D. et al. Differentiation of human NK cells into NK1 and NK2 subsets. J. Immunol. 161, 5821–5824 (1998).
Loza, M. J. & Perussia, B. Final steps of natural killer cell maturation: a model for type 1–type 2 differentiation? Nature Immunol. 2, 917–924 (2001).
Katsumoto, T. et al. STAT6-dependent differentiation and production of IL-5 and IL-13 in murine NK2 cells. J. Immunol. 173, 4967–4975 (2004).
Takahashi, K. et al. Natural killer type 2 bias in remission of multiple sclerosis. J. Clin. Invest. 107, R23–R29 (2001).
Bolay, H. et al. α Interferon treatment in myasthenia gravis: effects on natural killer cell activity. J. Neuroimmunol. 82, 109–115 (1998).
Hartrich, L. et al. Dynamics of immune cell trafficking in interferon-β treated multiple sclerosis patients. J. Neuroimmunol. 139, 84–92 (2003).
Chambers, B. J., Salcedo, M. & Ljunggren, H. G. Triggering of natural killer cells by the costimulatory molecule CD80 (B7-1). Immunity 5, 311–317 (1996). This was the first paper to show that NK cells can target autologous DCs.
Wilson, J. L. et al. Targeting of human dendritic cells by autologous NK cells. J. Immunol. 163, 6365–6370 (1999).
Andrews, D. M., Scalzo, A. A., Yokoyama, W. M., Smyth, M. J. & Degli-Esposti, M. A. Functional interactions between dendritic cells and NK cells during viral infection. Nature Immunol. 4, 175–181 (2003).
Piccioli, D., Sbrana, S., Melandri, E. & Valiante, N. M. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J. Exp. Med. 195, 335–341 (2002).
Gerosa, F. et al. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 195, 327–333 (2002).
Mailliard, R. B. et al. Dendritic cells mediate NK cell help for TH1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J. Immunol. 171, 2366–2373 (2003).
Carnaud, C. et al. Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163, 4647–4650 (1999).
Blanca, I. R., Bere, E. W., Young, H. A. & Ortaldo, J. R. Human B cell activation by autologous NK cells is regulated by CD40–CD40 ligand interaction: role of memory B cells and CD5+ B cells. J. Immunol. 167, 6132–6139 (2001).
Dowdell, K. C., Cua, D. J., Kirkman, E. & Stohlman, S. A. NK cells regulate CD4 responses prior to antigen encounter. J. Immunol. 171, 234–239 (2003).
Assarsson, E. et al. NK cells stimulate proliferation of T and NK cells through 2B4/CD48 interactions. J. Immunol. 173, 174–180 (2004).
Zingoni, A. et al. Cross-talk between activated human NK cells and CD4+ T cells via OX40–OX40 ligand interactions. J. Immunol. 173, 3716–3724 (2004).
Hanna, J. et al. Novel APC-like properties of human NK cells directly regulate T cell activation. J. Clin. Invest. 114, 1612–1623 (2004).
Trivedi, P. P., Roberts, P. C., Wolf, N. A. & Swanborg, R. H. NK cells inhibit T cell proliferation via p21-mediated cell cycle arrest. J. Immunol. 174, 4590–4597 (2005).
Gilmour, K. C. et al. Defective expression of the interleukin-2/interleukin-15 receptor β subunit leads to a natural killer cell-deficient form of severe combined immunodeficiency. Blood 98, 877–879 (2001).
Biron, C. A., Byron, K. S. & Sullivan, J. L. Severe herpes virus infections in an adolescent without natural killer cells. N. Engl. J. Med. 320, 1731–1735 (1989).
Zimmer, J., Bausinger, H. & De la Salle, H. Autoimmunity mediated by innate immune effector cells. Trends Immunol. 22, 300–301 (2001).
Moins-Teisserenc, H. T. et al. Association of a syndrome resembling Wegener's granulomatosis with low surface expression of HLA class-I molecules. Lancet 354, 1598–1603 (1999).
Markel, G. et al. The mechanisms controlling NK cell autoreactivity in TAP2-deficient patients. Blood 103, 1770–1778 (2004).
Kerschensteiner, M., Stadelmann, C., Dechant, G., Wekerle, H. & Hohlfeld, R. Neurotrophic cross-talk between the nervous and immune systems: implications for neurological diseases. Ann. Neurol. 53, 292–304 (2003).
Hammarberg, H. et al. Neuroprotection by encephalomyelitis: rescue of mechanically injured neurons and neurotrophin production by CNS-infiltrating T and natural killer cells. J. Neurosci. 20, 5283–5291 (2000).
Backstrom, E. et al. Natural killer cell-mediated lysis of dorsal root ganglia neurons via RAE1/NKG2D interactions. Eur. J. Immunol. 33, 92–100 (2003).
Morse, R. H., Seguin, R., McCrea, E. L. & Antel, J. P. NK cell-mediated lysis of autologous human oligodendrocytes. J. Neuroimmunol. 116, 107–115 (2001).
Antel, J. P., McCrea, E., Ladiwala, U., Qin, Y. F. & Becher, B. Non-MHC-restricted cell-mediated lysis of human oligodendrocytes in vitro: relation with CD56 expression. J. Immunol. 160, 1606–1611 (1998).
Trinchieri, G. Biology of natural killer cells. Adv. Immunol. 47, 187–376 (1989).
Shibatomi, K. et al. A novel role for interleukin-18 in human natural killer cell death: high serum levels and low natural killer cell numbers in patients with systemic autoimmune diseases. Arthritis Rheum. 44, 884–892 (2001).
Grom, A. A. et al. Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J. Pediatr. 142, 292–296 (2003).
Balandina, A., Lecart, S., Dartevelle, P., Saoudi, A. & Berrih-Aknin, S. Functional defect of regulatory CD4+CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood 105, 735–741 (2005).
Viglietta, V., Baecher-Allan, C., Weiner, H. L. & Hafler, D. A. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 199, 971–979 (2004).
La Cava, A., Van Kaer, L. & Shi, F.-D. CD4+CD25+ TRegs and NKT cells: regulators regulating regulators. Trends Immunol. 27, 322–327 (2006).
Ehl, S. et al. A comparison of efficacy and specificity of three NK depleting antibodies. J. Immunol. Methods 199, 149–153 (1996).
Ortaldo, J. R. & Young, H. A. Expression of IFN-γ upon triggering of activating Ly49D NK receptors in vitro and in vivo: costimulation with IL-12 or IL-18 overrides inhibitory receptors. J. Immunol. 170, 1763–1769 (2003).
Suzuki, H., Duncan, G. S., Takimoto, H. & Mak, T. W. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor β chain. J. Exp. Med. 185, 499–505 (1997).
Fehniger, T. A. & Caligiuri, M. A. Interleukin 15: biology and relevance to human disease. Blood 97, 14–32 (2001).
Lodolce, J. P. et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9, 669–676 (1998).
Kennedy, M. K. et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191, 771–780 (2000).
Takeda, K. et al. Defective NK cell activity and TH1 response in IL-18-deficient mice. Immunity 8, 383–390 (1998).
Adachi, O. et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9, 143–150 (1998).
Kim, S., Iizuka, K., Aguila, H. L., Weissman, I. L. & Yokoyama, W. M. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc. Natl Acad. Sci. USA 97, 2731–2736 (2000).
Townsend, M. J. et al. T-bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity 20, 477–494 (2004).
Flodstrom, M., Shi, F.-D., Sarvetnick, N. & Ljunggren, H. G. The natural killer cell — friend or foe in autoimmune disease? Scand. J. Immunol. 55, 432–441 (2002).
French, A. R. & Yokoyama, W. M. Natural killer cells and autoimmunity. Arthritis Res. Ther. 6, 8–14 (2004).
Johansson, S., Berg, L., Hall, H. & Hoglund, P. NK cells: elusive players in autoimmunity. Trends Immunol. 26, 613–618 (2005).
Maruyama, T. et al. Anti-asialo GM1 antibody suppression of cyclophosphamide-induced diabetes in NOD mice. Diabetes Res. 17, 37–41 (1991).
Fort, M. M., Leach, M. W. & Rennick, D. M. A role for NK cells as regulators of CD4+ T cells in a transfer model of colitis. J. Immunol. 161, 3256–3261 (1998).
Nilsson, N., Bremell, T., Tarkowski, A. & Carlsten, H. Protective role of NK1.1+ cells in experimental Staphylococcus aureus arthritis. Clin. Exp. Immunol. 117, 63–69 (1999).
Korsgren, M. et al. Natural killer cells determine development of allergen-induced eosinophilic airway inflammation in mice. J. Exp. Med. 189, 553–562 (1999).
Matsumoto, Y. et al. Role of natural killer cells and TCR γδ T cells in acute autoimmune encephalomyelitis. Eur. J. Immunol. 28, 1681–1688 (1998).
Zhang, B., Yamamura, T., Kondo, T., Fujiwara, M. & Tabira, T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J. Exp. Med. 186, 1677–1687 (1997).
Shi, F.-D. et al. Natural killer cells determine the outcome of B cell-mediated autoimmunity. Nature Immunol. 1, 245–251 (2000). This was the first study to indicate that NK cells have different roles in the initiation and the progression of autoimmune disease.
Johansson, S. E., Hall, H., Bjorklund, J. & Hoglund, P. Broadly impaired NK cell function in non-obese diabetic mice is partially restored by NK cell activation in vivo and by IL-12/IL-18 in vitro. Int. Immunol. 16, 1–11 (2004).
Ogasawara, K. et al. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity 20, 757–767 (2004).
Poirot, L., Benoist, C. & Mathis, D. Natural killer cells distinguish innocuous and destructive forms of pancreatic islet autoimmunity. Proc. Natl Acad. Sci. USA 101, 8102–8107 (2004).
Flodstrom, M. et al. Target cell defense prevents the development of diabetes after viral infection. Nature Immunol. 3, 373–382 (2002).
Lee, I. F., Qin, H., Trudeau, J., Dutz, J. & Tan, R. Regulation of autoimmune diabetes by complete Freund's adjuvant is mediated by NK cells. J. Immunol. 172, 937–942 (2004).
Paya, C. V., Patick, A. K., Leibson, P. J. & Rodriguez, M. Role of natural killer cells as immune effectors in encephalitis and demyelination induced by Theiler's virus. J. Immunol. 143, 95–102 (1989).
Xu, W., Fazekas, G., Hara, H. & Tabira, T. Mechanism of natural killer (NK) cell regulatory role in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 163, 24–30 (2005).
Smeltz, R. B., Wolf, N. A. & Swanborg, R. H. Inhibition of autoimmune T cell responses in the DA rat by bone marrow-derived NK cells in vitro: implications for autoimmunity. J. Immunol. 163, 1390–1397 (1999).
Shi, F.-D., Takeda, K., Akira, S., Sarvetnick, N. & Ljunggren, H. G. IL-18 directs autoreactive T cells and promotes autodestruction in the central nervous system via induction of IFN-γ by NK cells. J. Immunol. 165, 3099–3104 (2000). References 66–81 show that NK cells have an important role in several experimental autoimmune diseases. These studies also provide important insights into the mechanisms that are involved in these diseases.
Bettelli, E. et al. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J. Exp. Med. 200, 79–87 (2004).
Takeda, K. & Dennert, G. The development of autoimmunity in C57BL/6 lpr mice correlates with the disappearance of natural killer type 1-positive cells: evidence for their suppressive action on bone marrow stem cell proliferation, B cell immunoglobulin secretion, and autoimmune symptoms. J. Exp. Med. 177, 155–164 (1993).
Mendiratta, S. K. et al. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity 6, 469–477 (1997).
Kambayashi, T., Assarsson, E., Chambers, B. J. & Ljunggren, H. G. Expression of the DX5 antigen on CD8+ T cells is associated with activation and subsequent cell death or memory during influenza virus infection. Eur. J. Immunol. 31, 1523–1530 (2001).
Arase, H., Arase, N. & Saito, T. Interferon γ production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J. Exp. Med. 183, 2391–2396 (1996).
O'Garra, A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 8, 275–283 (1998).
Romagnani, S. Induction of TH1 and TH2 responses: a key role for the 'natural' immune response? Immunol. Today 13, 379–381 (1992).
Martin-Fontecha, A. et al. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nature Immunol. 5, 1260–1265 (2004).
Laouar, Y., Sutterwala, F. S., Gorelik, L. & Flavell, R. A. Transforming growth factor-β controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nature Immunol. 6, 600–607 (2005). References 89 and 90 provide insights into the mechanism of regulation of autoreactive T cells by cytokines produced by NK cells.
Zitvogel, L., Terme, M., Borg, C. & Trinchieri, G. Dendritic cell–NK cell cross-talk: regulation and physiopathology. Curr. Top. Microbiol. Immunol. 298, 157–174 (2006).
Banchereau, J., Pascual, V. & Palucka, A. K. Autoimmunity through cytokine-induced dendritic cell activation. Immunity 20, 539–550 (2004).
Fernandez, N. C. et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune response in vivo. Nature Med. 5, 405–411 (1999). This study shows the crosstalk that occurs between NK cells and DCs under pathophysiological conditions.
Charo, I. F. & Ransohoff, R. M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 (2006).
Salazar-Mather, T. P., Hamilton, T. A. & Biron, C. A. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J. Clin. Invest. 105, 985–993 (2000).
Campbell, J. J. et al. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J. Immunol. 166, 6477–6482 (2001).
Haskell, C. A. et al. Targeted deletion of CX3CR1 reveals a role for fractalkine in cardiac allograft rejection. J. Clin. Invest. 108, 679–688 (2001).
Huang, D. et al. CX3CR1 mediates specific recruitment to the central nervous system of NK cells that modify disease expression in experimental autoimmune encephalomyelitis. FASEB J. 20, 896–905 (2006). This study identifies CX 3 CL1 as the main chemoattractant for NK-cell homing to the CNS, a process that modulates the magnitude of EAE.
Fehniger, T. A. et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 101, 3052–3057 (2003). This paper shows that CD56hi NK cells are present in human lymph nodes and that endogenous T-cell-derived IL-2, binding the high-affinity IL-2 receptor at the surface of NK cells, co-stimulates CD56hi NK cells.
Liu, R. L. et al. Autoreactive T cells mediate NK cell degeneration in autoimmune disease. J. Immunol. 176, 5247–5254 (2006). This study shows that autoreactive T cells contribute to NK-cell degeneration and that NK-cell degeneration might have evolved to allow the immune system to control excessive autoimmunity.
Waldmann T. A., Dubois, S. & Tagaya, Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity 14, 105–110 (2001).
Koka, R. et al. Interleukin (IL)-15Rα-deficient natural killer cells survive in normal but not IL-15Rα-deficient mice. J. Exp. Med. 197, 977–984 (2003).
Cooper, M. A. et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood 100, 3633–3638 (2002).
Leonard, W. J. & Spolski, R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nature Rev. Immunol. 5, 688–698 (2005).
Kasaian, M. T. et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity 16, 559–569 (2002).
Vollmer, T. L. et al. Differential effects of IL-21 during initiation and progression of autoimmunity against neuroantigen. J. Immunol. 174, 2696–2701 (2005). References 105 and 106 show that the effect of IL-21 on NK-cell function depends on the status of NK-cell activation.
Vollmer, T. L. et al. Oral simvastatin treatment in relapsing–remitting multiple sclerosis. Lancet 363, 1607–1608 (2004).
Van Kaer, L. α-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nature Rev. Immunol. 5, 31–42 (2005).
Bielekova, B. et al. Regulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2R-targeted therapy (daclizumab) in multiple sclerosis. Proc. Natl Acad. Sci. USA 103, 5941–5946 (2006).
Li, Z., Lim, W. K., Mahesh, S. P., Liu, B. & Nussenblatt, R. B. In vivo blockade of human IL-2 receptor induces expansion of CD56bright regulatory NK cells in patients with active uveitis. J. Immunol. 174, 5187–5191 (2005).
Acknowledgements
We thank the following: R. M. Ransohoff for collaboration and crucial advice in preparation of this Review; T. Vollmer, A. La Cava, X. Bai, D. Huang and D. Campagnolo for fruitful discussions; and R. Liu, W. Piao and P. Minick for editorial assistance. We apologize to those colleagues whose work we could not cite as a result of space constraints. Work in the authors' laboratories is supported by the Muscular Dystrophy Association (USA), the Barrow Neurological Foundation (USA) and the National Institutes of Health (USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Negative selection
-
The deletion of self-reactive thymocytes, in the thymus. Thymocytes expressing T-cell receptors that strongly recognize self peptide bound to self MHC molecules undergo apoptosis in response to the signalling that results from high-affinity binding.
- Type 1 diabetes
-
A chronic autoimmune disease that is characterized by the T-cell-mediated destruction of β-cells (which secrete insulin), in the pancreas. Patients with type 1 diabetes develop hyperglycaemia and can develop diabetes-associated complications in multiple organ systems, owing to lack of insulin. Diabetes in non-obese diabetic (NOD) mice is a model of type 1 diabetes.
- Rheumatoid arthritis
-
A chronic systemic autoimmune disease that is mainly characterized by joint inflammation.
- Multiple sclerosis
-
A prevalent autoimmune disease of the brain and spinal cord that is characterized by inflammation, demyelination and axonal damage. The clinical presentation of neurological deficits is highly heterogeneous and depends on the site and the extent of central-nervous-system involvement.
- T helper 1 cell
-
(TH1 cell). CD4+ T cells are classified on the basis of the types of cytokine that they secrete. TH1 cells produce interferon-γ, lymphotoxin-α and tumour-necrosis factor, and they support cell-mediated immunity. TH2 cells produce interleukin-4 (IL-4), IL-5 and IL-13, and they support humoral immunity and downregulate TH1-cell responses. An imbalance between TH1-cell responses and TH2-cell responses is thought to contribute to the pathogenesis of various infections, allergic responses and autoimmune diseases.
- Natural killer T cells
-
(NKT cells). A heterogeneous subset of T cells, most of which express semi-invariant T-cell receptors. In mice, NKT cells were first identified through their expression of the cell-surface molecule natural-killer-cell-associated antigen 1.1 (NK1.1; also known as NKR-P1C).
- γδ T cell
-
A T cell that expresses a T-cell receptor consisting of a γ-chain and a δ-chain. These T cells are present mainly in the intestinal epithelium as intraepithelial lymphocytes (IELs). Although the exact function of γδ T cells (or IELs) is still unknown, it has been suggested that mucosal γδ T cells are involved in innate immune responses by the mucosal immune system.
- B1 cells
-
A group of self-renewing, autoreactive B cells with a limited B-cell-receptor repertoire. These cells are mainly found in the peritoneal cavity and the pleural cavity.
- Experimental allergic encephalomyelitis
-
(EAE). An animal model of multiple sclerosis. EAE can be induced in several mammalian species by immunization with myelin-derived antigens together with adjuvant. The immunized animals develop a paralytic disease that has several pathological features in common with multiple sclerosis in humans.
- Collagen-induced arthritis
-
An animal model of rheumatoid arthritis. Collagen-induced arthritis develops in susceptible rodents and primates after immunization with cartilage-derived type II collagen.
- Adjuvant
-
An agent that is mixed with an antigen and increases the immune response to immunization with that antigen.
- Type-II C-type lectin-like molecules
-
Lectins are carbohydrate-binding molecules, and C-type lectins were named after their ability to bind calcium. C-type lectin-like molecules — for example, many of the natural-killer-cell receptors — are disulphide-linked homodimers that have sequence homology to C-type lectins; however, they do not bind calcium, and they often recognize proteins instead of carbohydrates. Type II lectin-like molecules are type II transmembrane proteins, for which the amino terminus is intracellular and the carboxy terminus is extracellular.
- Transporter associated with antigen processing
-
(TAP). A molecule that translocates short peptides from the cytosol to the lumen of the endoplasmic reticulum, where these peptides can bind MHC class I molecules. TAP-deficient mice or humans have marked defects in cell-surface MHC class I expression, CD8+ T-cell development and natural killer (NK)-cell function. MHC-class-I-deficient cells are highly susceptible to lysis by wild-type NK cells, a finding that formed the basis for the 'missing-self' hypothesis of NK-cell-mediated target-cell recognition.
- Systemic lupus erythematosus
-
(SLE). A chronic systemic autoimmune disease that is characterized by rashes, arthritis, kidney disease and central-nervous-system involvement. It is mediated by antibodies that are specific for double-stranded DNA and other nuclear antigens.
- Myasthenia gravis
-
A chronic autoimmune disease that involves the generation of T-cell-dependent autoantibodies specific for the acetylcholine receptor. These antibodies interfere with the transmission of signals at neuromuscular junctions.
- CD4+CD25+ regulatory T cell
-
(TReg cell). A specialized type of CD4+ T cell that can suppress the responses of other T cells. These cells provide a crucial mechanism for the maintenance of peripheral self-tolerance and are characterized by expression of CD25 (also known as the α-chain of the interleukin-2 receptor) and the transcription factor forkhead box P3 (FOXP3).
Rights and permissions
About this article
Cite this article
Shi, FD., Van Kaer, L. Reciprocal regulation between natural killer cells and autoreactive T cells. Nat Rev Immunol 6, 751–760 (2006). https://doi.org/10.1038/nri1935
Issue Date:
DOI: https://doi.org/10.1038/nri1935
This article is cited by
-
Increased TIM-3+PD-1+ NK cells are associated with the disease activity and severity of systemic lupus erythematosus
Clinical and Experimental Medicine (2022)
-
Innate, innate-like and adaptive lymphocytes in the pathogenesis of MS and EAE
Cellular & Molecular Immunology (2019)
-
A kinetic model of T cell autoreactivity in autoimmune diseases
Journal of Mathematical Biology (2019)
-
Infiltration and persistence of lymphocytes during late-stage cerebral ischemia in middle cerebral artery occlusion and photothrombotic stroke models
Journal of Neuroinflammation (2017)
-
RNA Seq analysis for transcriptome profiling in response to classical swine fever vaccination in indigenous and crossbred pigs
Functional & Integrative Genomics (2017)