Key Points
-
Lyme arthritis was recognized as a separate entity from rheumatoid arthritis because of geographic clustering of children in Lyme, Connecticut, USA, who were thought to have juvenile rheumatoid arthritis.
-
The synovial histology in patients with chronic Lyme arthritis is typical of that found in all of the various forms of chronic inflammatory arthritis, including rheumatoid arthritis.
-
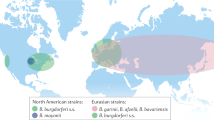
Lyme arthritis is now known to be a late manifestation of a tick-transmitted spirochetal infection, caused exclusively by Borrelia burgdorferi in the United States and mainly by Borrelia afzelii or Borrelia garinii in Europe and Asia. Of the three Borrelia species, B. burgdorferi is the most arthritogenic.
-
Studies in inbred strains of mice have delineated immune mechanisms that are important in susceptibility to and control of joint infection with B. burgdorferi.
-
Anti-inflammatory components of an innate immune response seem to protect some mouse strains from the development of severe arthritis despite infection of the joint.
-
Adaptive immune responses to B. burgdorferi control spirochetes and reduce the severity of arthritis in mice.
-
Lyme arthritis in human patients can usually be treated successfully with antibiotic therapy. However, in the United States, about 10% of patients develop persistent synovitis, which lasts for months or even several years after the apparent eradication of the spirochete from joints with antibiotic therapy.
-
Patients with antibiotic-treatment-resistant Lyme arthritis or rheumatoid arthritis share a similar immunogenetic susceptibility that mainly involves HLA-DRB1*0401, HLA-DRB1*0404 and HLA-DRB1*0101.
-
T-cell reactivity to an immunodominant epitope of B. burgdorferi outer-surface protein A (OspA) in genetically susceptible individuals is associated with treatment-resistant Lyme arthritis, but a relevant autoantigen has not been identified.
-
A crucial unresolved issue in Lyme arthritis is the nature of the stimulus that perpetuates synovial inflammation after the apparent eradication of live spirochetes from joints with antibiotic therapy.
Abstract
Before the first description of Lyme arthritis in 1976, patients with this disease were often thought to have juvenile or adult rheumatoid arthritis. It is now known that Lyme arthritis is caused by a tick-borne spirochete that disseminates to joints, where it induces marked pro-inflammatory responses. In most patients, the arthritis resolves with antibiotic treatment. However, in the United States, about 10% of patients with Lyme arthritis develop persistent synovitis, which lasts for months or even several years after the apparent eradication of the spirochete from the joint with antibiotic therapy. The elucidation of Lyme arthritis, from acute infection to chronic synovitis, might help in our understanding not only of this entity, but also of other forms of chronic inflammatory arthritis, including rheumatoid arthritis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Steere, A. C. et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum. 20, 7–17 (1977).
Steere, A. C. et al. Erythema chronicum migrans and Lyme arthritis: the enlarging clinical spectrum. Ann. Intern. Med. 86, 685–698 (1977).
Steere, A. C., Broderick, T. F. & Malawista, S. E. Erythema chronicum migrans and Lyme arthritis: epidemiologic evidence for a tick vector. Amer. J. Epidemiol. 108, 312–321 (1978).
Afzelius, A. Erythema chronicum migrans. Acta Derm. Venereol. (Stockh.) 2, 120–125 (1921).
Burgdorfer, W. et al. Lyme disease — a tick-borne spirochetosis? Science 216, 1317–1319 (1982).
Steere, A. C. et al. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308, 733–740 (1983).
Baranton, G. et al. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Internatl J. Systematic Bacteriol. 42, 378–383 (1992).
Steere, A. C., Schoen, R. T. & Taylor, E. The clinical evolution of Lyme arthritis. Ann. Intern. Med. 107, 725–731 (1987). This long-term longitudinal study presents the natural history of Lyme arthritis in patients before the use of antibiotics for treatment of the disorder.
Jaulhac, B. et al. Direct molecular typing of Borrelia burgdorferi sensu lato species in synovial samples from patients with Lyme arthritis. J. Clin. Microbiol 38, 1895–1900 (2000).
Lunemann, J. D. et al. Rapid typing of Borrelia burgdorferi sensu lato species in specimens from patients with different manifestations of Lyme borreliosis. J. Clin. Microbiol. 39, 1130–1133 (2001).
de Silva, A. M. & Fikrig, E. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J. Clin. Invest. 100, S3–S5 (1997).
Montgomery, R. R., Malawista, S. E., Feen, K. J. M. & Bockenstedt, L. K. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J. Exp. Med. 183, 261–269 (1996).
Schwan, T. G., Piesman, J., Golde, W. T., Dolan, M. C. & Rosa, P. A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl Acad. Sci. USA 92, 2909–2913 (1995).
Coleman, J. L. et al. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89, 1111–1119 (1997).
Seinost, G. et al. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect. Immun. 67, 3518–3524 (1999).
Zhang, J. -R. & Norris, S. J. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassettes-specific, segmental gene conversation. Infect. Immun. 66, 3698–3704 (1998).
Coburn, J., Chege, W., Magoun, L., Bodary, S. C. & Leong, J. M. Characterization of a candidate Borrelia burgdorferi β3-chain integrin ligand identified using a phage display library. Mol. Microbiol. 34, 926–940 (1999).
Parveen, N. & Leong, J. M. Identification of a candidate glycosaminoglycan-binding adhesin of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35, 1220–1234 (2000).
Probert, W. S. & Johnson, B. J. B. Identification of a 47kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B32. Mol. Microbiol. 30, 1003–1015 (1998).
Johnson, B. A., Haines, G. K., Harlow, L. A. & Koch, A. E. Adhesion molecule expression in human synovial tissue. Arthritis Rheum. 36, 137–146 (1993).
Guo, B. P., Brown, E. L., Dorward, D. W., Rosenberg, L. C. & Hook, M. Decorin-binding adhesins from Borrelia burgdorferi. Mol. Microbiol. 30, 711–723 (1998).
Duray, P. H. The surgical pathology of human Lyme disease: an enlarging picture. Am. J. Surg. Pathol. 11, 47–60 (1987).
Hefty, P. S., Jolliff, S. E., Caimano, M. J., Wikel, S. K. & Akins, D. R. Changes in temporal and spatial patterns of outer surface lipoprotein expression generate population heterogeneity and antigenic diversity in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 70, 3468–3478 (2002).
Muellegger, R. R. et al. Differential expression of cytokine mRNA in skin specimens from patients with erythema migrans or acrodermatitis chronica atrophicans. J. Invest. Dermatol. 115, 1115–1123 (2000).
Glickstein, L. et al. Inflammatory cytokine production predominates in early Lyme disease in patients with erythema migrans. Infect. Immun. 71, 6051–6053 (2003).
Salazar, J. C., et al. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J. Immunol. 171, 2660–2670 (2003).
Vaz, A. et al. Cellular and humoral immune responses to Borrelia burgdorferi antigens in patients with culture-positive early Lyme disease. Infect. Immun. 69, 7437–7444 (2001).
Akin, E., McHugh, G. L., Flavell, R. A., Fikrig, E. & Steere, A. C. The immunoglobin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect. Immun. 67, 173–181 (1999). This study gives the most complete analysis of the correlation of humoral immunity to outer-surface protein A (OspA) with treatment-resistant Lyme arthritis.
Nocton, J. J. et al. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid in Lyme arthritis. N. Engl. J. Med. 330, 229–234 (1994). This analysis of joint fluid samples from patients with Lyme arthritis shows that Borrelia burgdorferi DNA is usually present before antibiotic treatment, but not after ≥2 months of oral therapy or ≥1 month of intravenous therapy.
Hardin, J. A., Steere, A. C. & Malawista, S. E. Immune complexes and the evolution of Lyme arthritis: dissemination and localization of abnormal C1q binding activity. N. Engl. J. Med. 301, 1358–1363 (1979).
Steere, A. C., Hardin, J. A., Ruddy, S., Mummaw, J. G. & Malawista, S. E. Lyme arthritis: correlation of serum and cryoglobulin IgM with activity, and serum IgG with remission. Arthritis Rheum. 22, 471–483 (1979).
Beck, G., Benach, J. L. & Habicht, G. S. Isolation of interleukin 1 from joint fluids of patients with Lyme disease. J. Rheumatol. 16, 800–806 (1989).
Miller, L. C. et al. Balance of synovial fluid IL-1β and IL-1 receptor antagonist and recovery from Lyme arthritis. Lancet 341, 146–148 (1993).
Yin, Z. et al. T cell cytokine pattern in the joints of patients with Lyme arthritis and its regulation by cytokines and anticytokines. Arthritis Rheum. 40, 69–79 (1997).
Yssel, H. et al. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J. Exp. Med. 174, 593–601 (1991).
Gross, D. M., Steere, A. C. & Huber, B. T. T helper 1 response is dominant and localized to the synovial fluid in patients with Lyme arthritis. J. Immunol. 160, 1022–1028 (1998).
Roessner, K. et al. High expression of Fas ligand by synovial fluid-derived γδ T cells in Lyme arthritis. J. Immunol. 170, 2702–2710 (2003).
Glatzel, A. et al. The responsiveness of human Vδ1 γδ T cells to Borrelia burgdorferi is largely restricted to synovial-fluid cells from patients with Lyme arthritis. J. Infect. Dis. 186, 1043–1046 (2002).
Dressler, F., Whalen, J. A., Reinhardt, B. N. & Steere, A. C. Western blotting in the serodiagnosis of Lyme disease. J. Infect. Dis. 167, 392–400 (1993).
Steere, A. C. et al. Treatment of Lyme arthritis. Arthritis Rheum. 37, 878–888 (1994).
Barthold, S. W. et al. Experimental Lyme arthritis in rats infected with Borrelia burgdorferi. J. Infect. Dis. 157, 842–846 (1988).
Schaible, U. E. et al. The severe combined immunodeficiency (scid) mouse: a laboratory model for the analysis of Lyme arthritis and carditis. J. Exp. Med. 170, 1427–1432 (1989).
Barthold, S. W., Beck, D. S., Hansen, G. M., Terwilliger, G. A. & Moody, K. D. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162, 133–138 (1990).
Ma, Y. et al. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect. Immun. 66, 161–168 (1998).
Wooten, R. M. et al. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168, 348–355 (2002).
Alexopoulou, L. et al. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nature Med. 8, 878–884 (2002).
Jacchieri, S. G., et al. Structural study of binding of flagellin by Toll-like receptor 5. J. Bacteriol. 185, 4243–4247 (2003).
Talkington, J. & Nickell, S. P. Role of Fcγ receptors in triggering host cell activation and cytokine release by Borrelia burgdorferi. Infect. Immun. 69, 413–419 (2001).
Montgomery, R. R. & Malawista, S. E. Entry of Borrelia burgdorferi into macrophages is end-on and leads to degradation in lysosomes. Infect. Immun. 64, 2867–2872 (1996).
Rittig, M. G. et al. Coiling phagocytosis is the preferential phagocytic mechanism for Borrelia burgdorferi. Infect. Immun. 60, 4205–4212 (1992).
Modolell, M., Schaible, U. E., Rittig, M. & Simon, M. M. Killing of Borrelia burgdorferi by macrophages is dependent on oxygen radicals and nitric oxide and can be enhanced by antibodies to outer surface proteins of the spirochete. Immunol. Letters 40, 139–146 (1994).
Defosse, D. L. & Johnson, R. C. In vitro and in vivo induction of tumor necrosis factor α by Borrelia burgdorferi. Infect. Immun. 60, 1109–1113 (1992).
Anguita, J. et al. Cyclooxygenase 2 activity modulates the severity of murine Lyme arthritis. FEMS Immunol. Med. Microbiol. 34, 187–191 (2002).
Gebbia, J. A., Coleman, J. L. & Benach, J. L. Borrelia spirochetes upregulate release and activation of matrix metalloproteinase gelatinase B (MMP-9) and collagenase 1 (MMP-1) in human cells. Infect. Immun. 69, 456–462 (2001).
Brown, C. R., Blaho, V. A. & Loiacono, C. M. Susceptibility to experimental Lyme arthritis correlates with KC and monocytes chemoattractant protein-1 in joints and requires neutrophil recruitment via CXCR21. J. Immun. 171, 893–901 (2003).
Weis, J. J. et al. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J. Immunol. 162, 948–956 (1999). This study describes genetic linkage analyses for mice with heritable susceptibility to Lyme arthritis. It shows that arthritis consists of independent traits, such as swelling and leukocytic infiltrates, each with multigenic control.
Roper, R. J. et al. Genetic control of susceptibility to experimental Lyme arthritis is polygenic and exhibits consistent linkage to multiple loci on chromosome 5 in four independent mouse crosses. Genes Immun. 2, 388–397 (2001).
Brown, C. R. & Reiner, S. L. Genetic control of experimental Lyme arthritis in the absence of specific immunity. Infect. Immun. 67, 1967–1973 (1999). This paper shows conclusively that spirochete expansion is controlled by adaptive T- and B-cell immune responses, whereas arthritis is controlled by innate immune factors that differ between C3H and C57BL/6 mouse strains.
McKisic, M. D. & Barthold, S. W. T-cell-independent responses to Borrelia burgdorferi are critical for protective immunity and resolution of Lyme disease. Infect. Immun. 68, 5190–5197 (2000).
McKisic, M. D., Redmond, W. L. & Barthold, S. W. Cutting edge: T cell-mediated pathology in murine Lyme borreliosis. J. Immunol. 164, 6096–6099 (2000).
Barthold, S. W. & de Souza, M. Exacerbation of Lyme arthritis in beige mice. J. Infect. Dis. 172, 778–784 (1995).
Brown, J. P., Zachary, J. F., Teuscher, C., Weis, J. J. & Wooten, R. M. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect. Immun. 67, 5142–5150 (1999).
Anguita, J. et al. Borrelia burgdorferi-infected, interleukin-6-deficient mice have decreased TH2 responses and increased Lyme arthritis. J. Infect. Dis. 178, 1512–1515 (1998).
Radolf, J. D. et al. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J. Immunol. 154, 2866–2877 (1995).
Erdile, L. F. et al. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect. Immun. 61, 81–90 (1993).
Fikrig, E., Barthold, S. W., Kantor, F. S. & Flavell, R. A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science 250, 553–556 (1990).
Nguyen, T. P. et al. Partial destruction of Borrelia burgdorferi within ticks that engorged on OspE- or OspF-immunized mice. Infect. Immun. 62, 2079–2084 (1994).
Fikrig, E., Barthold, S. W., Chen, M., Chang, C. H. & Flavell, R. A. Protective antibodies develop, and murine Lyme arthritis regresses, in the absence of MHC class II and CD4+ T cells. J. Immunol. 159, 5682–5686 (1997).
Fikrig, E. et al. Protective antibodies in murine Lyme disease arise independently of CD40 ligand. J. Immunol. 157, 1–3 (1996).
McKisic, M. D. & Barthold, S. W. T-cell-independent responses to Borrelia burgdorferi are critical for protective immunity and resolution of Lyme disease. Infect. Immun. 68, 5190–5197 (2000).
Fikrig, E. et al. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity 6, 531–539 (1997).
Hanson, M. S. et al. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect. Immun. 66, 2143–2153 (1998).
Feng, S., Hodzic, E. & Barthold, S. W. Lyme arthritis resolution with antiserum to a 37-kilodalton Borrelia burgdorferi protein. Infect. Immun. 68, 4169–4173 (2000). This study identifies a single B. burgdorferi protein (Arp1) that induces an antibody response, which reduces the severity of arthritis in mice despite ongoing infection.
Keane-Myers, A. & Nickell, S. P. T cell subset-dependent modulation of immunity to Borrelia burgdorferi in mice. J. Immunol. 154, 1770–1776 (1995).
Dong, Z., Edelstein, M. D. & Glickstein, L. J. CD8+ T cells are activated during the early TH1 and TH2 immune responses in a murine Lyme disease model. Infect. Immun. 65, 5334–5337 (1997).
Zeidner, N. et al. Effects of Ixodes scapularis and Borrelia burgdorferi on modulation of the host immune response: induction of a TH2 cytokine response in Lyme disease-susceptible (C3H/HeJ) mice but not in disease-resistant (BALB/c) mice. Infect. Immun. 65, 3100–3106 (1997).
Kang, I., Barthold, S. W., Persing, D. H. & Bockenstedt, L. K. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect. Immun. 65, 3107–3111 (1997).
Schaible, U. E. et al. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manifests predominantly in the joints, heart, and liver. Am. J. Pathol. 137, 811–820 (1990).
Barthold, S. W., Sidman, C. L. & Smith, A. L. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am. J. Trop. Med. Hyg. 47, 605–613 (1992).
Lawson, J. P. & Steere, A. C. Lyme arthritis: radiographic findings. Radiology 154, 37–43 (1985).
Dattwyler, R. J., Halperin, J. J., Volkman, D. J. & Luft, B. J. Treatment of late Lyme borreliosis – randomized comparison of ceftriaxone and penicillin. Lancet 1, 1191–1194 (1988).
Malawista, S. E. Resolution of Lyme arthritis, acute or prolonged: a new look. Inflammation 24, 493–504 (2000).
Girschick, H. J., Huppertz, H. I., Rûssmann, H., Krenn, V. & Karch, H. Intracellular persistence of Borrelia burgdorferi in human synovial cells. Rheumatol. Internatl 16, 125–132 (1996).
Franz, J. K. et al. Insights from a novel three-dimensional in vitro model of Lyme arthritis. Arthritis Rheum. 44, 151–162 (2001).
Fikrig, E. et al. An OspA frame shift, identified from DNA in Lyme arthritis synovial fluid, results in an outer surface protein A that does not bind protective antibodies. J. Immunol. 155, 5700–5704 (1995).
Kalish, R. A., Leong, J. M. & Steere, A. C. Association of treatment resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect. Immun. 61, 2774–2779 (1993).
Priem, S. et al. Detection of Borrelia burgdoferi by polymerase chain reaction in synovial membrane, but not in synovial fluid from patients with persisting Lyme arthritis after antibiotic therapy. Ann. Rheum. Dis. 57, 118–121 (1998). This article shows that B. burgdorferi DNA might sometimes be detected in synovial tissue after it is no longer found in joint fluid.
Carlson, D. et al. Lack of Borrelia burgdorferi DNA in synovial samples in patients with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum. 42, 2705–2709 (1999).
Bockenstedt, L. K., Mao, J., Hodzic, E., Barthold, S. W. & Fish, D. Detection of attenuated, noninfectious spirochetes in Borrelia burgdorferi-infected mice after antibiotic treatment. J. Infect. Dis. 186, 1430–1437 (2002).
Gondolf, K. B., Mihatsch, M., Curschellas, E., Dunn, J. J. & Batsford, S. R. Induction of experimental allergic arthritis with outer surface proteins of Borrelia burgdorferi. Arthritis Rheum. 37, 1070–1077 (1994).
Steere, A. C., Dwyer, E. & Winchester, R. Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 alleles. N. Engl. J. Med. 323, 219–223 (1990).
Steere, A. C. et al. Binding of outer surface protein A and human lymphocyte function- associated antigen 1 peptides to HLA-DR molecules associated with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum. 48, 534–540 (2003). This study shows that the strength of binding of OspA peptides to HLA-DR molecules correlates with the frequency of these HLA-DR alleles in patients with treatment-resistant Lyme arthritis.
Gregerson, P. K., Silver, J. & Winchester, R. J. The shared epitope hypothesis: an approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 30, 1205–1213 (1987).
Weyand, C. M., McCarthy, T. G. & Goronzy, J. J. The influence of HLA-DRB1 genes on disease severity in rheumatoid arthritis. Ann. Intern. Med. 117, 801–806 (1992).
Nepom, B. S. & Glass, D. N. Juvenile rheumatoid arthritis and HLA: report of the Park City III workshop. J. Rheumatol. Suppl. 33, 70–74 (1992).
Reimers, C. D., Neubert, U., Krisoferitsch, W., Pfluger, K. H. & Mayr, W. R. Borrelia burgdorferi infection in Europe: an HLA-related disease? Infection 20, 197–200 (1992).
Kalish, R. A., Leong, J. M. & Steere, A. C. Early and late antibody responses to full-length and truncated constructs of outer-surface protein A of Borrelia burgdorferi in Lyme disease. Infect. Immun. 63, 2228–2235 (1995).
Lengl-Janssen, B., Strauss, A. F., Steere, A. C. & Kamradt, T. The T helper cell response in Lyme arthritis: differential recognition of Borrelia burgdorferi outer surface protein A (OspA) in patients with treatment-resistant or treatment-responsive Lyme arthritis. J. Exp. Med. 180, 2069–2078 (1994).
Chen, J. et al. Association of antibiotic treatment-resistant Lyme arthritis with T cell responses to dominant epitopes of outer-surface protein A (OspA) of Borrelia burgdorferi. Arthritis Rheum. 42, 1813–1822 (1999). This study links T-cell responses to dominant epitopes of OspA with treatment-resistant Lyme arthritis.
Crowley, H. & Huber, B. T. Host-adapted Borrelia burgdorferi in mice expresses OspA during inflammation. Infect. Immun. 71, 4003–4010 (2003).
Olsson, I., Asbrink, E., Von Stedingk, M. & Von Stedingk, L. V. Changes in Borrelia burgdorferi-specific serum IgG antibody levels in patients treated for acrodermatitis chronica atrophicans. Acta Derm. Venereol. 74, 424–428 (1994).
Gross, D. M. et al. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science 281, 703–706 (1998). This is the original study that presents the concept of molecular mimicry in patients with treatment-resistant Lyme arthritis.
Steere, A. C., Gross, D., Meyer, A. L. & Huber, B. T. Autoimmune mechanisms in antibiotic treatment-resistant Lyme arthritis. J. Autoimmun. 16, 263–268 (2001).
Meyer, A. L. et al. Direct enumeration of Borrelia-reactive CD4+ T cells ex vivo by using MHC class II tetramers. Proc. Natl Acad. Sci. USA 97, 11433–11438 (2000).
Limbach, F. X. et al. Treatment resistant Lyme arthritis caused by Borrelia garinii. Ann. Rheum. Dis. 60, 284–286 (2001).
Firestein, G. S. Evolving concepts of rheumatoid arthritis. Nature 423, 356–361 (2003).
Firestein, G. S. & Zvaifler, N. J. How important are T cells in chronic rheumatoid synovitis. Arthritis Rheum. 46, 298–308 (2002).
Trollmo, C., Meyer, A. L., Steere, A. C., Hafler, D. A. & Huber, B. T. Molecular mimicry in Lyme arthritis demonstrated at the single cell level: LFA-1αL is a partial agonist for outer surface protein A-reactive T cells. J. Immunol. 166, 5286–5291 (2001). This study presents data that concern the concept of molecular mimicry in patients with treatment-resistant Lyme arthritis.
Kalish, R. S. et al. Human T lymphocyte response to Borrelia burgdorferi infection: no correlation between human leukocyte function antigen type 1 peptide response and clinical status. J. Infect. Dis. 187, 102–108 (2003).
Maier, B. et al. Multiple cross-reactive self-ligands for Borrelia burgdorferi-specific HLA-DR4-restricted T cells. Eur. J. Immunol. 30, 448–457 (2000).
Benoist, C. & Mathis, D. Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nature Immunol. 2, 797–801 (2001). This article explores several mechanisms by which infection might trigger autoimmunity including T-cell epitope mimicry and bystander activation of autoreactive T cells.
Johnston, Y. E. et al. Lyme arthritis: spirochetes found in synovial microangiopathic lesions. Am. J. Pathol. 118, 26–34 (1985)
Steere, A. C., Duray, P. H. & Butcher, E. C. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis: comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 31, 487–495 (1988).
Akin, E., Aversa, J. & Steere, A. C. Expression of adhesion molecules in synovia of patients with treatment-resistant Lyme arthritis. Infect. Immun. 69, 1774–1780 (2001).
Harjacek, M. et al. Prominent expression of mRNA for proinflammatory cytokines in synovium in patients with juvenile rheumatoid arthritis or chronic Lyme arthritis. J. Rheumatol. 27, 497–503 (2000).
Lin, B. et al. Differences in synovial fluid levels of matrix metalloproteinases suggest separate mechanisms of pathogenesis in Lyme arthritis before and after antibiotic treatment. J. Infect. Dis. 184, 174–180 (2001).
Matsumoto, I., Staub, A., Benoist, C. & Mathis, D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science 286, 1732–1735 (1999).
Acknowledgements
This work was supported in part by the National Institutes of Health, the Mathers Foundation, the English, Bonter, Mitchell Foundation, the Lyme/Arthritis Reserach Fund and the Eshe Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- ADULT RHEUMATOID ARTHRITIS
-
A form of chronic inflammatory arthritis that affects many large and small joints symmetrically. In early rheumatoid arthritis, only a few joints might be affected initially, and patients with Lyme arthritis were sometimes thought to have early seronegative (rheumatoid-factor negative) rheumatoid arthritis.
- JUVENILE RHEUMATOID ARTHRITIS
-
(JRA). An inflammatory arthritis in children that lasts for at least six weeks, which is not caused by other known types of arthritis. In the pauciarticular form of the disease, which is most similar to Lyme arthritis, only one or a few joints are affected, most commonly the knee.
- REACTIVE ARTHRITIS
-
A sterile joint inflammation that develops after a distant infection. The arthritis might affect only a few joints, including the knee, and might last for months to several years, as in Lyme arthritis.
- SYNOVIAL TISSUE
-
A thin, highly vascular lining tissue that covers all intra-articular structures except for articular cartilage. It secretes small amounts of joint fluid, which might function as a lubricant and a source of nutrients for the relatively avascular cartilage in the joint.
- LYME DISEASE
-
A complex, multi-system infection that is caused by a tick-transmitted spirochetal bacterium, Borrelia burgdorferi. After the initial infection of the skin (stage 1), the spirochete often disseminates to many sites (stage 2), particularly to other skin sites, the nervous system, heart or joints, where it might cause persistent infection (stage 3) for years.
- ACRODERMATITIS CHRONICA ATROPHICANS
-
A late skin manifestation of Borrelia afzelii infection that mainly occurs in older patients, most often on distal surfaces of the extremities. The inflammatory phase is followed by thinning of the skin, a process that might continue for decades.
Rights and permissions
About this article
Cite this article
Steere, A., Glickstein, L. Elucidation of Lyme arthritis. Nat Rev Immunol 4, 143–152 (2004). https://doi.org/10.1038/nri1267
Issue Date:
DOI: https://doi.org/10.1038/nri1267
This article is cited by
-
Lyme arthritis: linking infection, inflammation and autoimmunity
Nature Reviews Rheumatology (2021)
-
Biologic Markers of Antibiotic-Refractory Lyme Arthritis in Human: A Systematic Review
Infectious Diseases and Therapy (2019)
-
Metabolites of prostaglandin synthases as potential biomarkers of Lyme disease severity and symptom resolution
Inflammation Research (2019)
-
When a patient suspected with juvenile idiopathic arthritis turns out to be diagnosed with an infectious disease – a review of Lyme arthritis in children
Pediatric Rheumatology (2017)
-
Lyme borreliosis
Nature Reviews Disease Primers (2016)