Abstract
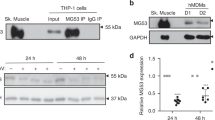
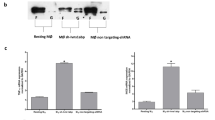
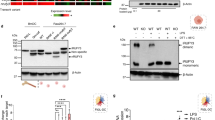
Vimentin is a widely expressed intermediate filament protein thought to be involved mainly in structural processes, such as wound healing1. We now demonstrate that activated human macrophages secrete vimentin into the extracellular space. The maturation of blood-derived monocytes into macrophages involves several signalling pathways2,3. We show that secretion of vimentin, which is phosphorylated at serine and threonine residues, is enhanced by the phosphatase inhibitor okadaic acid and blocked by the specific protein kinase C inhibitor GÖ6983. These findings are consistent with previous observations that phosphorylation of vimentin affects its intracellular localization and that vimentin is a substrate for protein kinase C (PKC)4,5. We also show that the anti-inflammatory cytokine interleukin-10 (IL-10), which inhibits PKC activity, blocks secretion of vimentin. In contrast, the pro-inflammatory cytokine tumour necrosis factor α (TNF-α) can trigger secretion of vimentin. Finally, we found that extracellular vimentin is involved in bacterial killing and the generation of oxidative metabolites, two important functions of activated macrophages. These data establish that vimentin is secreted by macrophages in response to pro-inflammatory signalling pathways and is probably involved in immune function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Eckes, B. et al. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J. Cell Sci. 111, 1897–1907 (1998).
Reddy, V. Y., Zhang, Q. Y. & Weiss, S. J. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc. Natl Acad. Sci. USA 92, 3849–3853 (1995).
Punturieri, A. et al. Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K-deficient human macrophages. J. Exp. Med. 192, 789–800 (2000).
Turowski, P., Myles, T., Hemmings, B. A., Fernandez, A. & Lamb, N. J. Vimentin dephosphorylation by protein phosphatase 2A is modulated by the targeting subunit B55. Mol. Biol. Cell 10, 1997–2015 (1999).
Yasui, Y. et al. Protein kinases required for segregation of vimentin filaments in mitotic process. Oncogene 20, 2868–2876 (2001).
Traub, P. Intermediate Filaments: A Review (Springer-Verlag, New York, 1985).
Christian, J. L., Edelstein, N. G. & Moon, R. T. Overexpression of wild-type and dominant negative mutant vimentin subunits in developing Xenopus embryos. New Biol. 2, 700–711 (1990).
Eckes, B. et al. Impaired wound healing in embryonic and adult mice lacking vimentin. J. Cell Sci. 113, 2455–2462 (2000).
Cain, H., Kraus, B., Krauspe, R., Osborn, M. & Weber, K. Vimentin filaments in peritoneal macrophages at various stages of differentiation and with altered function. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 42, 65–81 (1983).
Rius, C., Cabanas, C. & Aller, P. The induction of vimentin gene expression by sodium butyrate in human promonocytic leukemia U937 cells. Exp. Cell Res. 188, 129–134 (1990).
Rius, C. & Aller, P. Vimentin expression as a late event in the in vitro differentiation of human promonocytic cells. J. Cell Sci. 101, 395–401 (1992).
Cain, H., Krauspe, R. & Kraus, B. The cytoskeleton in activated and in functionally disordered cells of the macrophage system. Pathol. Res. Pract. 175, 162–79 (1982).
Gao, Y. & Sztul, E. A novel interaction of the Golgi complex with the vimentin intermediate filament cytoskeleton. J. Cell Biol. 152, 877–894 (2001).
Cheng, T. J. & Lai, Y. K. Identification of mitogen-activated protein kinase-activated protein kinase-2 as a vimentin kinase activated by okadaic acid in 9L rat brain tumor cells. J. Cell Biochem. 71, 169–181 (1998).
Owen, P. J., Johnson, J. D. & Lord, J. M. Protein kinase Cδ associates with vimentin intermediate filaments in differentiated HL60 cells. Exp. Cell Res. 225, 366–373 (1996).
Lo, C.-J., Fu, M. & Cryer, H. G. Interleukin 10 inhibits alveolar macrophage production of inflammatory mediators involved in adult respiratory distress Syndrome. J. Surgical Res. 79, 179–184 (1998).
Bhattacharyya, S., Ghosh, S., Jhonson, P. L., Bhattacharya, S. K. & Majumdar, S. Immunomodulatory role of interleukin-10 in visceral leishmaniasis: defective activation of protein kinase C-mediated signal transduction events. Infect. Immun. 69, 1499–1507 (2001).
Bogdan, C., Vodovotz, Y. & Nathan, C. Macrophage deactivation by interleukin 10. J. Exp. Med. 174, 1549–1555 (1991).
Schlosser-Silverman, E., Elgrably-Weiss, M., Rosenshine, I., Kohen, R. & Altuvia, S. Characterization of Escherichia coli DNA lesions generated within J774 macrophages. J. Bacteriol. 182, 5225–5230 (2000).
Perides, G., Harter, C. & Traub, P. Electrostatic and hydrophobic interactions of the intermediate filament protein vimentin and its amino terminus with lipid bilayers. J. Biol. Chem. 262, 13742–13749 (1987).
Nishimura, N. & Balch, W. E. A di-acidic signal required for selective export from the endoplasmic reticulum. Science 277, 556–558 (1997).
Nishimura, N. et al. A di-acidic (DXE) code directs concentration of cargo during export from the endoplasmic reticulum. J. Biol. Chem. 274, 15937–15946 (1999).
Hansson, G. K., Starkebaum, G. A., Benditt, E. P. & Schwartz, S. M. Fc-mediated binding of IgG to vimentin-type intermediate filaments in vascular endothelial cells. Proc. Natl Acad. Sci. USA 81, 3103–3107 (1984).
Hansson, G. K., Lagerstedt, E., Bengtsson, A. & Heideman, M. IgG binding to cytoskeletal intermediate filaments activates the complement cascade. Exp. Cell Res. 170, 338–350 (1987).
Sanchez, A., Ossorio, C., Alvaro-Gracia, J. M., Padilla, R. & Avila, J. A subset of antibodies from the sera of patients with systemic lupus erythematosus react with vimentin and DNA. J. Rheumatol. 17, 205–209 (1990).
Senecal, J. L. & Rauch, J. Hybridoma lupus autoantibodies can bind major cytoskeletal filaments in the absence of DNA-binding activity. Arthritis Rheumatol. 31, 864–875 (1988).
Franch, A., Castellote, C., Vila, J. L., Vilaro, S. & Castell, M. Anticytoskeletal autoantibody development in adjuvant arthritis. J. Rheumatol. 21, 489–497 (1994).
Terasaki, M. & Reese, T. S. Characterization of endoplasmic reticulum by co-localization of BiP and dicarbocyanine dye. J. Cell Sci. 101, 315–322 (1992).
Matsukawa, A. et al. Pivotal role of the CC chemokine, macrophage-derived chemokine, in the innate immune response. J. Immunol. 164, 5362–5268 (2000).
Acknowledgements
We thank S. Weiss and B. Lane for technical advice and intellectual support, B. Donohoe for help in generating the microscopic images, M. Swanson for help with the phagocytosis assay and J. Cleary for advice on the manuscript. This work was supported by grants to D.M.M. from the American Cancer Society and the Arthritis Foundation, the Rheumatic Disease Core Center of the University of Michigan (5 P30 AR48310-02) and the General Clinical Research Center at the University of Michigan (M01-RR00042). N.M.-V. was supported by a grant from the Arthritis Foundation. A.P. was supported by Merit Review funding and a Research Enhancement Awards Program (REAP) grant from the Department of Veterans Affairs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
We have filed a patent application on the role of secreted vimentin in immunity.
Supplementary information
Supplementary Figures and Table
Figure S1. Vimentin is secreted via the classical ER/Golgi pathway. (PDF 807 kb)
Figure S2. The specificity of vimentin antibodies.
Figure S3. Vimentin is phosphorylated on serine/threonine residues.
Table 1. Isolation and partial amino acid sequence analysis of vimentin from the supernatant of MDM
Rights and permissions
About this article
Cite this article
Mor-Vaknin, N., Punturieri, A., Sitwala, K. et al. Vimentin is secreted by activated macrophages. Nat Cell Biol 5, 59–63 (2003). https://doi.org/10.1038/ncb898
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb898
This article is cited by
-
Methacrylic Acid-Based Regenerative Biomaterials: Explorations into the MAAgic
Regenerative Engineering and Translational Medicine (2023)
-
Circulating Vimentin Over-Expression in Patients with Oral Sub Mucosal Fibrosis and Oral Squamous Cell Carcinoma
Indian Journal of Otolaryngology and Head & Neck Surgery (2022)
-
SARS-CoV-2: Receptor and Co-receptor Tropism Probability
Current Microbiology (2022)
-
Molecular and cellular immune features of aged patients with severe COVID-19 pneumonia
Communications Biology (2022)
-
Molecular characterization and cell type composition deconvolution of fibrosis in NAFLD
Scientific Reports (2021)