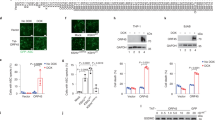
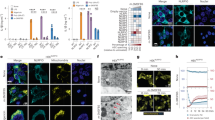
Abstract
Host- and pathogen-associated cytoplasmic double-stranded DNA triggers the activation of a NALP3 (also known as cryopyrin and NLRP3)-independent inflammasome1, which activates caspase-1 leading to maturation of pro-interleukin-1β and inflammation. The nature of the cytoplasmic-DNA-sensing inflammasome is currently unknown. Here we show that AIM2 (absent in melanoma 2), an interferon-inducible HIN-200 family member that contains an amino-terminal pyrin domain and a carboxy-terminal oligonucleotide/oligosaccharide-binding domain2,3, senses cytoplasmic DNA by means of its oligonucleotide/oligosaccharide-binding domain and interacts with ASC (apoptosis-associated speck-like protein containing a CARD) through its pyrin domain to activate caspase-1. The interaction of AIM2 with ASC also leads to the formation of the ASC pyroptosome4, which induces pyroptotic cell death in cells containing caspase-1. Knockdown of AIM2 by short interfering RNA reduced inflammasome/pyroptosome activation by cytoplasmic DNA in human and mouse macrophages, whereas stable expression of AIM2 in the non-responsive human embryonic kidney 293T cell line conferred responsiveness to cytoplasmic DNA. Our results show that cytoplasmic DNA triggers formation of the AIM2 inflammasome by inducing AIM2 oligomerization. This study identifies AIM2 as an important inflammasome component that senses potentially dangerous cytoplasmic DNA, leading to activation of the ASC pyroptosome and caspase-1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Muruve, D. A. et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452, 103–107 (2008)
Choubey, D. & Panchanathan, R. Interferon-inducible Ifi200-family genes in systemic lupus erythematosus. Immunol. Lett. 119, 32–41 (2008)
Ludlow, L. E., Johnstone, R. W. & Clarke, C. J. The HIN-200 family: more than interferon-inducible genes? Exp. Cell Res. 308, 1–17 (2005)
Fernandes-Alnemri, T. et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14, 1590–1604 (2007)
Petrilli, V., Dostert, C., Muruve, D. A. & Tschopp, J. The inflammasome: a danger sensing complex triggering innate immunity. Curr. Opin. Immunol. 19, 615–622 (2007)
Halle, A. et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nature Immunol. 9, 857–865 (2008)
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nature Immunol. 9, 847–856 (2008)
Yu, J. W. et al. Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol. Cell 28, 214–227 (2007)
Yu, J. W. et al. Cryopyrin and pyrin activate caspase-1, but not NF-κB, via ASC oligomerization. Cell Death Differ. 13, 236–249 (2006)
Agostini, L. et al. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle–Wells autoinflammatory disorder. Immunity 20, 319–325 (2004)
Albrecht, M., Choubey, D. & Lengauer, T. The HIN domain of IFI-200 proteins consists of two OB folds. Biochem. Biophys. Res. Commun. 327, 679–687 (2005)
DeYoung, K. L. et al. Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene 15, 453–457 (1997)
Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008)
Eisenbarth, S. C., Colegio, O. R., O’Connor, W., Sutterwala, F. S. & Flavell, R. A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126 (2008)
Franchi, L. & Nunez, G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1β secretion but dispensable for adjuvant activity. Eur. J. Immunol. 38, 2085–2089 (2008)
Meier, O., Gastaldelli, M., Boucke, K., Hemmi, S. & Greber, U. F. Early steps of clathrin-mediated endocytosis involved in phagosomal escape of Fcγ receptor-targeted adenovirus. J. Virol. 79, 2604–2613 (2005)
Srinivasula, S. M. et al. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J. Biol. Chem. 277, 21119–21122 (2002)
Fernandes-Alnemri, T. & Alnemri, E. S. Chapter thirteen assembly, purification, and assay of the activity of the ASC pyroptosome. Methods Enzymol. 442, 251–270 (2008)
Acknowledgements
This work was supported by NIH grants AG14357 and AR055398 to E.S.A. We thank M. McCormick and D. Wang for technical assistance, X. Jiao for help with the confocal microscopy, J. Sagara for the anti-human ASC antibody, J. Yuan for the anti-mouse caspase-1 antibody, R. Johnstone for the anti-human AIM2 antibody, M.-C. Hung for the anti-human IFIX antibody, T. Ouchi for the IFI16 complementary DNA and E. Latz for the immortalized mouse bone marrow macrophages.
Author Contributions T.F.-A. and J.-W.Y. performed most of the experiments. J.W. and P.D. performed additional experiments and provided technical assistance with equal contribution. E.S.A. conceived and designed the experiments, analysed and interpreted the data (with T.F.-A. and J.-W.Y.), and directed the whole project.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Notes, Supplementary Figures 1-15 with Legends, Legend for Supplementary Movie 1, a Supplementary Discussion and Supplementary References (PDF 3137 kb)
Supplementary Movie 1
Supplementary Movie 1 is a. time-lapse live cell movie of cytoplasmic DNA-induced AIM2 oligomerization and pyroptosis in Nalp3-/- bone marrow macrophages. (see nature 07710-s1 for full Legend) (AVI 939 kb)
Rights and permissions
About this article
Cite this article
Fernandes-Alnemri, T., Yu, JW., Datta, P. et al. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009). https://doi.org/10.1038/nature07710
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07710
This article is cited by
-
Inflammasomes in neurological disorders — mechanisms and therapeutic potential
Nature Reviews Neurology (2024)
-
The role of inflammasomes in human diseases and their potential as therapeutic targets
Signal Transduction and Targeted Therapy (2024)
-
Deficiency of circadian clock gene Bmal1 exacerbates noncanonical inflammasome-mediated pyroptosis and lethality via Rev-erbα-C/EBPβ-SAA1 axis
Experimental & Molecular Medicine (2024)
-
Hippocampal Microglia Activation Induced by Acute Pancreatic Injury in Rats
Digestive Diseases and Sciences (2024)
-
Candida albicans extracellular vesicles trigger type I IFN signalling via cGAS and STING
Nature Microbiology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.