Abstract
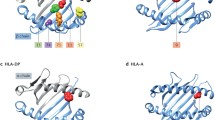
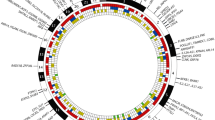
The “Bermuda triangle” of genetics, environment and autoimmunity is involved in the pathogenesis of rheumatoid arthritis (RA). Various aspects of genetic contribution to the etiology, pathogenesis and outcome of RA are discussed in this review. The heritability of RA has been estimated to be about 60 %, while the contribution of HLA to heritability has been estimated to be 11–37 %. Apart from known shared epitope (SE) alleles, such as HLA-DRB1*01 and DRB1*04, other HLA alleles, such as HLA-DRB1*13 and DRB1*15 have been linked to RA susceptibility. A novel SE classification divides SE alleles into S1, S2, S3P and S3D groups, where primarily S2 and S3P groups have been associated with predisposition to seropositive RA. The most relevant non-HLA gene single nucleotide polymorphisms (SNPs) associated with RA include PTPN22, IL23R, TRAF1, CTLA4, IRF5, STAT4, CCR6, PADI4. Large genome-wide association studies (GWAS) have identified more than 30 loci involved in RA pathogenesis. HLA and some non-HLA genes may differentiate between anti-citrullinated protein antibody (ACPA) seropositive and seronegative RA. Genetic susceptibility has also been associated with environmental factors, primarily smoking. Some GWAS studies carried out in rodent models of arthritis have confirmed the role of human genes. For example, in the collagen-induced (CIA) and proteoglycan-induced arthritis (PgIA) models, two important loci — Pgia26/Cia5 and Pgia2/Cia2/Cia3, corresponding the human PTPN22/CD2 and TRAF1/C5 loci, respectively — have been identified. Finally, pharmacogenomics identified SNPs or multiple genetic signatures that may be associated with responses to traditional disease-modifying drugs and biologics.
Similar content being viewed by others
References
Alamanos Y, Drosos AA (2005) Epidemiology of adult rheumatoid arthritis. Autoimmun Rev 4(3):130–136
Klareskog L, Padyukov L, Alfredsson L (2007) Smoking as a trigger for inflammatory rheumatic diseases. Curr Opin Rheumatol 19(1):49–54
van der Helm-van Mil AH, Wesoly JZ, Huizinga TW (2005) Understanding the genetic contribution to rheumatoid arthritis. Curr Opin Rheumatol 17(3):299–304
van der Woude D, Alemayehu WG, Verduijn W, de Vries RR, Houwing-Duistermaat JJ, Huizinga TW et al (2010) Gene–environment interaction influences the reactivity of autoantibodies to citrullinated antigens in rheumatoid arthritis. Nat Genet 42(10):814–816, author reply 816
Szodoray P, Szabo Z, Kapitany A, Gyetvai A, Lakos G, Szanto S et al (2010) Anti-citrullinated protein/peptide autoantibodies in association with genetic and environmental factors as indicators of disease outcome in rheumatoid arthritis. Autoimmun Rev 9(3):140–143
de Vries R (2011) Genetics of rheumatoid arthritis: time for a change! Curr Opin Rheumatol 23(3):227–232
Cooles FA, Isaacs JD. Pathophysiology of rheumatoid arthritis. Curr Opin Rheumatol;23(3):233–40
Szekanecz Z, Soos L, Szabo Z, Fekete A, Kapitany A, Vegvari A et al (2008) Anti-citrullinated protein antibodies in rheumatoid arthritis: as good as it gets? Clin Rev Allergy Immunol 34(1):26–31
Klareskog L, Padyukov L, Lorentzen J, Alfredsson L (2006) Mechanisms of disease: genetic susceptibility and environmental triggers in the development of rheumatoid arthritis. Nat Clin Pract Rheumatol 2(8):425–433
Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L (2004) A gene–environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum 50(10):3085–3092
Smolen JS, Landewe R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C et al (2010) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 69(6):964–975
MacGregor AJ, Snieder H, Rigby AS, Koskenvuo M, Kaprio J, Aho K et al (2000) Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum 43(1):30–37
Mesko B, Poliska S, Szegedi A, Szekanecz Z, Palatka K, Papp M et al (2010) Peripheral blood gene expression patterns discriminate among chronic inflammatory diseases and healthy controls and identify novel targets. BMC Med Genomics 3:15
Lee HS, Irigoyen P, Kern M, Lee A, Batliwalla F, Khalili H et al (2007) Interaction between smoking, the shared epitope, and anti-cyclic citrullinated peptide: a mixed picture in three large North American rheumatoid arthritis cohorts. Arthritis Rheum 56(6):1745–1753
Kapitany A, Szabo Z, Lakos G, Aleksza M, Vegvari A, Soos L et al (2008) Associations between serum anti-CCP antibody, rheumatoid factor levels and HLA-DR4 expression in Hungarian patients with rheumatoid arthritis. Isr Med Assoc J 10(1):32–36
Besenyei T, Gyetvai A, Szabo Z, Fekete A, Kapitany A, Szodoray P et al (2011) Associations of HLA-shared epitope, anti-citrullinated peptide antibodies and lifestyle-related factors in Hungarian patients with rheumatoid arthritis: data from the first Central-Eastern European cohort. Joint Bone Spine 78(6):652–653
Scott IC, Steer S, Lewis CM, Cope AP (2011) Precipitating and perpetuating factors of rheumatoid arthritis immunopathology: linking the triad of genetic predisposition, environmental risk factors and autoimmunity to disease pathogenesis. Best Pract Res Clin Rheumatol 25(4):447–468
Vittecoq O, Lequerre T, Goeb V, Le Loet X, Abdesselam TA, Klemmer N (2008) Smoking and inflammatory diseases. Best Pract Res Clin Rheumatol 22(5):923–935
Davila L, Ranganathan P (2011) Pharmacogenetics: implications for therapy in rheumatic diseases. Nat Rev Rheumatol 7(9):537–550
Cronstein BN (2006) Pharmacogenetics in the rheumatic diseases, from pret-a-porter to haute couture. Nat Clin Pract Rheumatol 2(1):2–3
Danila MI, Hughes LB, Bridges SL (2008) Pharmacogenetics of etanercept in rheumatoid arthritis. Pharmacogenomics 9(8):1011–1015
Mesko B, Poliska S, Szamosi S, Szekanecz Z, Podani J, Varadi C et al (2012) Peripheral blood gene expression and IgG glycosylation profiles as markers of tocilizumab treatment in rheumatoid arthritis. J Rheumatol 39(5):916–928
Plant D, Bowes J, Potter C, Hyrich KL, Morgan AW, Wilson AG et al (2011) Genome-wide association study of genetic predictors of anti-tumor necrosis factor treatment efficacy in rheumatoid arthritis identifies associations with polymorphisms at seven loci. Arthritis Rheum 63(3):645–653
Centola M, Szekanecz Z, Kiss E, Zeher M, Szegedi G, Nakken B et al (2007) Gene expression profiles of systemic lupus erythematosus and rheumatoid arthritis. Expert Rev Clin Immunol 3(5):797–806
Feng T, Zhu X (2010) Genome-wide searching of rare genetic variants in WTCCC data. Hum Genet 128(3):269–280
Craddock N, Hurles ME, Cardin N, Pearson RD, Plagnol V, Robson S et al (2010) Genome-wide association study of CNVs in 16,000 cases of eight common diseases and 3,000 shared controls. Nature 464(7289):713–720
Adarichev VA, Vermes C, Hanyecz A, Mikecz K, Bremer EG, Glant TT (2005) Gene expression profiling in murine autoimmune arthritis during the initiation and progression of joint inflammation. Arthritis Res Ther 7(2):R196–R207
Ahlqvist E, Hultqvist M, Holmdahl R (2009) The value of animal models in predicting genetic susceptibility to complex diseases such as rheumatoid arthritis. Arthritis Res Ther 11(3):226
Glant TT, Finnegan A, Mikecz K (2003) Proteoglycan-induced arthritis: immune regulation, cellular mechanisms, and genetics. Crit Rev Immunol 23(3):199–250
Glant TT, Mikecz K, Arzoumanian A, Poole AR (1987) Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis Rheum 30(2):201–212
Glant TT, Adarichev VA, Nesterovitch AB, Szanto S, Oswald JP, Jacobs JJ et al (2004) Disease-associated qualitative and quantitative trait loci in proteoglycan-induced arthritis and collagen-induced arthritis. Am J Med Sci 327(4):188–195
Deighton CM, Walker DJ, Griffiths ID, Roberts DF (1989) The contribution of HLA to rheumatoid arthritis. Clin Genet 36(3):178–182
Gregersen PK, Silver J, Winchester RJ (1987) The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum 30(11):1205–1213
van der Woude D, Houwing-Duistermaat JJ, Toes RE, Huizinga TW, Thomson W, Worthington J et al (2009) Quantitative heritability of anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis. Arthritis Rheum 60(4):916–923
van der Helm-van Mil AH, Verpoort KN, le Cessie S, Huizinga TW, de Vries RR, Toes RE (2007) The HLA-DRB1 shared epitope alleles differ in the interaction with smoking and predisposition to antibodies to cyclic citrullinated peptide. Arthritis Rheum 56(2):425–432
du Montcel ST, Michou L, Petit-Teixeira E, Osorio J, Lemaire I, Lasbleiz S et al (2005) New classification of HLA-DRB1 alleles supports the shared epitope hypothesis of rheumatoid arthritis susceptibility. Arthritis Rheum 52(4):1063–1068
van der Woude D, Lie BA, Lundstrom E, Balsa A, Feitsma AL, Houwing-Duistermaat JJ et al (2010) Protection against anti-citrullinated protein antibody-positive rheumatoid arthritis is predominantly associated with HLA-DRB1*1301: a meta-analysis of HLA-DRB1 associations with anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis in four European populations. Arthritis Rheum 62(5):1236–1245
Laki J, Lundstrom E, Snir O, Ronnelid J, Ganji I, Catrina AI et al (2012) Very high levels of anti-citrullinated protein antibodies are associated with HLA-DRB1*15 non-shared epitope allele in patients with rheumatoid arthritis. Arthritis Rheum 64(7):2078–2084
Zsilak S, Gal J, Hodinka L, Rajczy K, Balog A, Sipka S et al (2005) HLA-DR genotypes in familial rheumatoid arthritis: increased frequency of protective and neutral alleles in a multicase family. J Rheumatol 32(12):2299–2302
Jawaheer D, Thomson W, MacGregor AJ, Carthy D, Davidson J, Dyer PA et al (1994) "Homozygosity" for the HLA-DR shared epitope contributes the highest risk for rheumatoid arthritis concordance in identical twins. Arthritis Rheum 37(5):681–686
Gyetvai A, Szekanecz Z, Soos L, Szabo Z, Fekete A, Kapitany A, et al. New classification of the shared epitope in rheumatoid arthritis: impact on the production of various anti-citrullinated protein antibodies. Rheumatology (Oxford) 2009
Huizinga TW, Amos CI, van der Helm-van Mil AH, Chen W, van Gaalen FA, Jawaheer D et al (2005) Refining the complex rheumatoid arthritis phenotype based on specificity of the HLA-DRB1 shared epitope for antibodies to citrullinated proteins. Arthritis Rheum 52(11):3433–3438
Bax M, van Heemst J, Huizinga TW, Toes RE (2011) Genetics of rheumatoid arthritis: what have we learned? Immunogenetics 63(8):459–466
Farago B, Magyari L, Safrany E, Csongei V, Jaromi L, Horvatovich K et al (2008) Functional variants of interleukin-23 receptor gene confer risk for rheumatoid arthritis but not for systemic sclerosis. Ann Rheum Dis 67(2):248–250
Farago B, Talian GC, Komlosi K, Nagy G, Berki T, Gyetvai A et al (2009) Protein tyrosine phosphatase gene C1858T allele confers risk for rheumatoid arthritis in Hungarian subjects. Rheumatol Int 29(7):793–796
Stahl EA, Raychaudhuri S, Remmers EF, Xie G, Eyre S, Thomson BP et al (2010) Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet 42(6):508–514
Goeb V, Dieude P, Daveau R, Thomas-L'otellier M, Jouen F, Hau F et al (2008) Contribution of PTPN22 1858T, TNFRII 196R and HLA-shared epitope alleles with rheumatoid factor and anti-citrullinated protein antibodies to very early rheumatoid arthritis diagnosis. Rheumatology (Oxford) 47(8):1208–1212
Cha S, Choi CB, Han TU, Kang CP, Kang C, Bae SC (2007) Association of anti-cyclic citrullinated peptide antibody levels with PADI4 haplotypes in early rheumatoid arthritis and with shared epitope alleles in very late rheumatoid arthritis. Arthritis Rheum 56(5):1454–1463
Poor G, Nagy ZB, Schmidt Z, Brozik M, Meretey K, Gergely P Jr (2007) Genetic background of anticyclic citrullinated peptide autoantibody production in Hungarian patients with rheumatoid arthritis. Ann N Y Acad Sci 1110:23–32
Suzuki A, Yamada R, Chang X, Tokuhiro S, Sawada T, Suzuki M et al (2003) Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet 34(4):395–402
van der Linden MP, Feitsma AL, le Cessie S, Kern M, Olsson LM, Raychaudhuri S et al (2009) Association of a single-nucleotide polymorphism in CD40 with the rate of joint destruction in rheumatoid arthritis. Arthritis Rheum 60(8):2242–2247
Liang YL, Wu H, Shen X, Li PQ, Yang XQ, Liang L, et al. Association of STAT4 rs7574865 polymorphism with autoimmune diseases: a meta-analysis. Mol Biol Rep 2012.
Plenge RM, Seielstad M, Padyukov L, Lee AT, Remmers EF, Ding B et al (2007) TRAF1-C5 as a risk locus for rheumatoid arthritis—a genomewide study. N Engl J Med 357(12):1199–1209
Lee YH, Ji JD, Song GG (2008) Associations between FCGR3A polymorphisms and susceptibility to rheumatoid arthritis: a metaanalysis. J Rheumatol 35(11):2129–2135
Kochi Y, Okada Y, Suzuki A, Ikari K, Terao C, Takahashi A et al (2010) A regulatory variant in CCR6 is associated with rheumatoid arthritis susceptibility. Nat Genet 42(6):515–519
Szekanecz Z, Koch AE, Tak PP (2011) Chemokine and chemokine receptor blockade in arthritis, a prototype of immune-mediated inflammatory diseases. Neth J Med 69(9):356–366
Ding B, Padyukov L, Lundstrom E, Seielstad M, Plenge RM, Oksenberg JR et al (2009) Different patterns of associations with anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis in the extended major histocompatibility complex region. Arthritis Rheum 60(1):30–38
Verpoort KN, Cheung K, Ioan-Facsinay A, van der Helm-van Mil AH, de Vries-Bouwstra JK, Allaart CF et al (2007) Fine specificity of the anti-citrullinated protein antibody response is influenced by the shared epitope alleles. Arthritis Rheum 56(12):3949–3952
Daha NA, Toes RE (2011) Rheumatoid arthritis: Are ACPA-positive and ACPA-negative RA the same disease? Nat Rev Rheumatol 7(4):202–203
Pedersen M, Jacobsen S, Klarlund M, Pedersen BV, Wiik A, Wohlfahrt J et al (2006) Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res Ther 8(4):R133
Perricone C, Ceccarelli F, Valesini G. An overview on the genetic of rheumatoid arthritis: a never-ending story. Autoimmun Rev, 10(10):599–608
Ruyssen-Witrand A, Rouanet S, Combe B, Dougados M, Le Loet X, Sibilia J et al (2012) Fcgamma receptor type IIIA polymorphism influences treatment outcomes in patients with rheumatoid arthritis treated with rituximab. Ann Rheum Dis 71(6):875–877
van Ede AE, Laan RF, Blom HJ, Huizinga TW, Haagsma CJ, Giesendorf BA et al (2001) The C677T mutation in the methylenetetrahydrofolate reductase gene: a genetic risk factor for methotrexate-related elevation of liver enzymes in rheumatoid arthritis patients. Arthritis Rheum 44(11):2525–2530
Berkun Y, Levartovsky D, Rubinow A, Orbach H, Aamar S, Grenader T et al (2004) Methotrexate related adverse effects in patients with rheumatoid arthritis are associated with the A1298C polymorphism of the MTHFR gene. Ann Rheum Dis 63(10):1227–1231
Dervieux T, Kremer J, Lein DO, Capps R, Barham R, Meyer G et al (2004) Contribution of common polymorphisms in reduced folate carrier and gamma-glutamylhydrolase to methotrexate polyglutamate levels in patients with rheumatoid arthritis. Pharmacogenetics 14(11):733–739
Pawlik A, Wrzesniewska J, Fiedorowicz-Fabrycy I, Gawronska-Szklarz B (2004) The MDR1 3435 polymorphism in patients with rheumatoid arthritis. Int J Clin Pharmacol Ther 42(9):496–503
Tolusso B, Pietrapertosa D, Morelli A, De Santis M, Gremese E, Farina G et al (2006) IL-1B and IL-1RN gene polymorphisms in rheumatoid arthritis: relationship with protein plasma levels and response to therapy. Pharmacogenomics 7(5):683–695
van Vollenhoven RF (2007) Switching between anti-tumour necrosis factors: trying to get a handle on a complex issue. Ann Rheum Dis 66(7):849–851
Acknowledgments
This work was supported by research grants ETT 315/2009 from the Medical Research Council of Hungary (Z.S.); by the TÁMOP 4.2.1/B-09/1/KONV-2010-0007 and TÁMOP-4.2.2.A-11/1/KONV-2012-0031 projects co-financed by the European Union and the European Social Fund (Z.S.), the Bridging Fund provided by the University of Debrecen, Medical and Health Sciences Center (Z.S.), and a grant (R01 AR059356) awarded by the National Institutes of Health, USA (T.T.G.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurkó, J., Besenyei, T., Laki, J. et al. Genetics of Rheumatoid Arthritis — A Comprehensive Review. Clinic Rev Allerg Immunol 45, 170–179 (2013). https://doi.org/10.1007/s12016-012-8346-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-012-8346-7