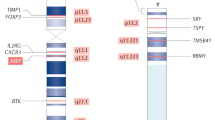
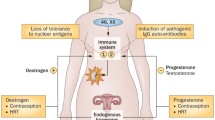
Abstract
Despite recent progress in the understanding of systemic lupus erythematosus (SLE), the striking 9:1 female to male ratio of disease incidence remains largely unexplained. In addition, peak SLE incidence rates occur during the early reproductive years in women. Studies which illuminate potential causes underlying this sex difference and characteristic onset during the reproductive years have the potential to fundamentally advance our understanding of disease pathogenesis in SLE. Similarly, progress in this area will likely inform human reproductive immunology. Studies of sex hormone function in the immune system are of obvious importance; however, it seems likely that many other types of sex-related genetic and immunological differences will contribute to SLE. In this review, we will focus on recent work in sex-related differences in cytokine pathways and genetics of these pathways as they relate to SLE pathogenesis. It seems quite possible that many of these sex-related differences could be important to reproductive fitness, which may explain the conservation of these immune system features and the observed female predominance of SLE.

Similar content being viewed by others
References
Lupus Foundation of America (www.lupus.org). In: 2009
Petri M (2002) Epidemiology of systemic lupus erythematosus. Best Pract Res Clin Rheumatol 16(5):847–858
Rus V, Maury EE, Hochberg MC (eds) (2002) The epidemiology of systemic lupus erythematosus. Williams and Wilkins, Philadelphia
Chakravarty EF, Bush TM, Manzi S, Clarke AE, Ward MM (2007) Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum 56(6):2092–2094
Lopez P, Mozo L, Gutierrez C, Suarez A (2003) Epidemiology of systemic lupus erythematosus in a northern Spanish population: gender and age influence on immunological features. Lupus 12(11):860–865
Bazer FW STE, Johnson GA, Burghardt RC, Wu G (2009) Comparative aspects of implantation. Reproduction 138(2):195–209
Szyper-Kravitz M, Zandman-Goddard G, Lahita RG, Shoenfeld Y (2005) The neuroendocrine-immune interactions in systemic lupus erythematosus: a basis for understanding disease pathogenesis and complexity. Rheum Dis Clin North Am 31(1):161–175, x
Stevens AM, Tsao BP, Hahn BH, Guthrie K, Lambert NC, Porter AJ et al (2005) Maternal HLA class II compatibility in men with systemic lupus erythematosus. Arthritis Rheum 52(9):2768–2773
Chagnon P, Schneider R, Hebert J, Fortin PR, Provost S, Belisle C et al (2006) Identification and characterization of an Xp22.33;Yp11.2 translocation causing a triplication of several genes of the pseudoautosomal region 1 in an XX male patient with severe systemic lupus erythematosus. Arthritis Rheum 54(4):1270–1278
Sawalha AH, Webb R, Han S, Kelly JA, Kaufman KM, Kimberly RP et al (2008) Common variants within MECP2 confer risk of systemic lupus erythematosus. PLoS ONE 3(3):e1727
Jacob CO, Zhu J, Armstrong DL, Yan M, Han J et al (2009) Identification of IRAK1 as a risk gene with critical role in the pathogenesis of systemic lupus erythematosus. PNAS 106(15):6256–6261
Forton AC, Petri MA, Goldman D, Sullivan KE (2002) An osteopontin (SPP1) polymorphism is associated with systemic lupus erythematosus. Hum Mutat 19(4):459
D'Alfonso S, Barizzone N, Giordano M, Chiocchetti A, Magnani C, Castelli L et al (2005) Two single-nucleotide polymorphisms in the 5′ and 3′ ends of the osteopontin gene contribute to susceptibility to systemic lupus erythematosus. Arthritis Rheum 52(2):539–547
Han S, Guthridge JM, Harley IT, Sestak AL, Kim-Howard X, Kaufman KM et al (2008) Osteopontin and systemic lupus erythematosus association: a probable gene–gender interaction. PLoS ONE 3(3):e0001757
Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J (2001) Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science 294(5546):1540–1543
Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL (1979) Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med 301(1):5–8
Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK (2005) Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum 52(5):1491–1503
Ronnblom LE, Alm GV, Oberg KE (1990) Possible induction of systemic lupus erythematosus by interferon-alpha treatment in a patient with a malignant carcinoid tumour. J Intern Med 227(3):207–210
Niewold TB, Swedler WI (2005) Systemic lupus erythematosus arising during interferon-alpha therapy for cryoglobulinemic vasculitis associated with hepatitis C. Clin Rheumatol 24(2):178–181
Niewold TB (2008) Interferon alpha-induced lupus: proof of principle. J Clin Rheumatol 14(3):131–132
Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK (2007) High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun 8:492–502
Niewold TB, Adler JE, Glenn SB, Lehman TJ, Harley JB, Crow MK (2008) Age- and sex-related patterns of serum interferon-alpha activity in lupus families. Arthritis Rheum 58(7):2113–2119
Kariuki SN, Niewold TB. Genetic regulation of serum cytokines in systemic lupus erythematosus. Transl Res 2009, In press
Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK (2008) Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum 58(8):2481–2487
Salloum R, Franek BS, Kariuki SN, Rhee L, Mikolaitis RA, Jolly M et al Genetic variation at the IRF7/PHRF1 locus is associated with autoantibody profile and serum interferon alpha activity in lupus patients. Arthritis Rheum 2009, In press
Kariuki SN, Crow MK, Niewold TB (2008) The PTPN22 C1858T polymorphism is associated with skewing of cytokine profiles toward high interferon-alpha activity and low tumor necrosis factor alpha levels in patients with lupus. Arthritis Rheum 58(9):2818–2823
Kariuki SN, Moore JG, Kirou KA, Crow MK, Utset TO, Niewold TB (2009) Age- and gender-specific modulation of serum osteopontin and interferon-alpha by osteopontin genotype in systemic lupus erythematosus. Genes Immun 10(5):487–494
Kariuki SN, Kirou KA, MacDermott EJ, Barillas-Arias L, Crow MK, Niewold TB (2009) Cutting edge: autoimmune disease risk variant of STAT4 confers increased sensitivity to IFN-alpha in lupus patients in vivo. J Immunol 182(1):34–38
Pestka S, Krause CD, Walter MR (2004) Interferons, interferon-like cytokines, and their receptors. Immunol Rev 202:8–32
Bazer FW WG, Spencer TE, Johnson GA, Burghardt RC, Bayless K. Novel Pathways for Implantation and Establishment and Maintenance of Pregnancy in Mammals. Mol Hum Reprod 2009
Johnson GA, Bazer FW, Burghardt RC, Spencer TE, Wu G, Bayless KJ (2009) Conceptus-uterus interactions in pigs: endometrial gene expression in response to estrogens and interferons from conceptuses. Soc Reprod Fertil 66(Suppl):321–332
Peeva E, Grimaldi C, Spatz L, Diamond B (2000) Bromocriptine restores tolerance in estrogen-treated mice. J Clin Invest 106(11):1373–1379
Hughes GC, Clark EA (2007) Regulation of dendritic cells by female sex steroids: relevance to immunity and autoimmunity. Autoimmunity 40(6):470–481
Siracusa MC, Overstreet MG, Housseau F, Scott AL, Klein SL (2008) 17beta-estradiol alters the activity of conventional and IFN-producing killer dendritic cells. J Immunol 180(3):1423–1431
Hughes GC, Thomas S, Li C, Kaja MK, Clark EA (2008) Cutting edge: progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. J Immunol 180(4):2029–2033
Lahita RG (1999) The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol 11(5):352–356
Doria A, Cutolo M, Ghirardello A, Zampieri S, Vescovi F, Sulli A et al (2002) Steroid hormones and disease activity during pregnancy in systemic lupus erythematosus. Arthritis Rheum 47(2):202–209
Nalbandian G, Kovats S (2005) Understanding sex biases in immunity: effects of estrogen on the differentiation and function of antigen-presenting cells. Immunol Res 31(2):91–106
Zandman-Goddard G, Peeva E, Shoenfeld Y (2007) Gender and autoimmunity. Autoimmun Rev 6(6):366–372
Drexler SK, Foxwell BM (2009) The role of Toll-like receptors in chronic inflammation. Int J Biochem Cell Biol
Ronnblom L, Alm GV, Eloranta ML (2009) Type I interferon and lupus. Curr Opin Rheumatol 21(5):471–477
Izui S (1990) Autoimmune accelerating genes, lpr and Yaa, in murine systemic lupus erythematosus. Autoimmunity 6(1–2):113–129
Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J et al (2006) A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci USA 103(26):9970–9975
Pisitkun P, Deane J, Difilippantonio M, Tarasenko T, Satterthwaite A, Bolland S (2006) Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 312:1669–1672
Berghofer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H (2006) TLR7 ligands induce higher IFN-alpha production in females. J Immunol 177(4):2088–2096
Visentini M, Conti V, Cagliuso M, Tinti F, Siciliano G, Trombetta AC et al (2009) Regression of systemic lupus erythematosus after development of an acquired Toll-like receptor signaling defect and antibody deficiency. Arthritis Rheum 60(9):2767–2771
Kelley J, Johnson MR, Alarcon GS, Kimberly RP, Edberg JC (2007) Variation in the relative copy number of the TLR7 gene in patients with systemic lupus erythematosus and healthy control subjects. Arthritis Rheum 56(10):3375–3378
Jimenez SA, Artlett CM (2005) Microchimerism and systemic sclerosis. Curr Opin Rheumatol 17(1):86–90
Lambert NC, Stevens AM, Tylee TS, Erickson TD, Furst DE, Nelson JL (2001) From the simple detection of microchimerism in patients with autoimmune diseases to its implication in pathogenesis. Ann NY Acad Sci 945:164–171
Johnson KL, McAlindon TE, Mulcahy E, Bianchi DW (2001) Microchimerism in a female patient with systemic lupus erythematosus. Arthritis Rheum 44(9):2107–2111
Kremer Hovinga IC, Koopmans M, Grootscholten C, van der Wal AM, Bijl M, Derksen RH et al (2008) Pregnancy, chimerism and lupus nephritis: a multi-centre study. Lupus 17(6):541–547
Reed A, Picnorell YJ, Harwood A, Kredich D (2000) Chimerism in children with juvenile dermatomyositis. Lancet 356:2156–2157
Artlett CM, Ramos R, Jiminez SA, Patterson K, Miller FW, Rider LG, the Childhood Myositis Heterogeneity Collaborative Group (2000) Chimeric cells of maternal origin in juvenile idiopathic inflammatory myopathies. Lancet 356:2155–2156
Stevens AM, Hermes HM, Rutledge JC, Buyon JP, Nelson JL (2000) Myocardial-tissue-specific phenotype of maternal microchimerism in neonatal lupus congenital heart block. Lancet 362:1617–1623
Walsh RJ, Kong SW, Yao Y, Jallal B, Kiener PA, Pinkus JL et al (2007) Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum 56(11):3784–3792
Niewold TB, Kariuki SN, Morgan GA, Shrestha S, Pachman LM (2009) Elevated serum interferon-alpha activity in juvenile dermatomyositis: associations with disease activity at diagnosis and after thirty-six months of therapy. Arthritis Rheum 60(6):1815–1824
Niewold TB, Rivera TL, Buyon JP, Crow MK (2008) Serum type I interferon activity is dependent on maternal diagnosis in anti-SSA/Ro-positive mothers of children with neonatal lupus. Arthritis Rheum 58(2):541–546
Stevens AM (2006) Microchimeric cells in systemic lupus erythematosus: targets or innocent bystanders? Lupus 15(11):820–826
Larizza D, Calcaterra V, Martinetti M (2009) Autoimmune stigmata in Turner syndrome: when lacks an X chromosome. J Autoimmun 33(1):25–30
Invernizzi P, Miozzo M, Selmi C, Persani L, Battezzati P, Zuin M et al (2005) X chromosome monosomy: a common mechanism for autoimmune disease. J Immunol 175:575–578
Invernizzi P, Miozzo M, Oertelt-Prigione S, Meroni PL, Persani L, Selmi C et al (2007) X monosomy in female systemic lupus erythematosus. Ann NY Acad Sci 1110:84–91
Cooney CM, Bruner GR, Aberle T, Namjou-Khales B, Myers LK, Feo L et al (2009) 46, X, del(X)(q13) Turner's syndrome women with systemic lupus erythematosus in a pedigree multiplex for SLE. Genes Immun 10(5):478–481
Scofield RH, Bruner GR, Namjou B, Kimberly RP, Ramsey-Goldman R, Petri M et al (2008) Klinefelter's syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum 58(8):2511–2517
Ozbalkan Z, Bagislar S, Kiraz S, Akyerli CB, Ozer HT, Yavuz S et al (2005) Skewed X chromosome inactivation in blood cells of women with scleroderma. Arthritis Rheum 52(5):1564–1570
Ozcelik T, Uz E, Akyerli CB, Bagislar S, Mustafa CA, Gursoy A et al (2006) Evidence from autoimmune thyroiditis of skewed X-chromosome inactivation in female predisposition to autoimmunity. Eur J Hum Genet 14(6):791–797
Invernizzi P, Pasini S, Selmi C, Miozzo M, Podda M (2008) Skewing of X chromosome inactivation in autoimmunity. Autoimmunity 41(4):272–277
Chitnis S, Monteiro J, Glass D, Apatoff B, Salmon J, Concannon P et al (2000) The role of X-chromosome inactivation in female predisposition to autoimmunity. Arthritis Res 2:399–406
Pan Y, Sawalha AH (2009) Epigenetic regulation and the pathogenesis of systemic lupus erythematosus. Transl Res 153(1):4–10
Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH et al (2006) Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol 7(5):498–506
Masutani K, Akahoshi M, Tsuruya K, Tokumoto M, Ninomiya T, Kohsaka T et al (2001) Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum 44(9):2097–2106
Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT et al (2001) The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 294(5547):1731–1735
Denhardt DT, Noda M (1998) Osteopontin expression and function: role in bone remodeling. J Cell Biochem Suppl 30–31:92–102
Vanacker JM, Delmarre C, Guo X, Laudet V (1998) Activation of the osteopontin promoter by the orphan nuclear receptor estrogen receptor related alpha. Cell Growth Differ 9(12):1007–1014
Author information
Authors and Affiliations
Corresponding author
Additional information
C. E. Weckerle has no grant support or disclosures to declare. Grant support for and disclosures by T. B. Niewold include NIH K08 AI083790, NIAID Clinical Research Loan Repayment AI071651, Arthritis National Research Foundation Eng Tan Scholar Award, Lupus Research Institute Novel Research Grant, University of Chicago CTSA Core Subsidy Grant, and Collaborative University of Chicago/Northshore University Health System Translational Research Pilot Grant from UL1 RR024999.
Rights and permissions
About this article
Cite this article
Weckerle, C.E., Niewold, T.B. The Unexplained Female Predominance of Systemic Lupus Erythematosus: Clues from Genetic and Cytokine Studies. Clinic Rev Allerg Immunol 40, 42–49 (2011). https://doi.org/10.1007/s12016-009-8192-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-009-8192-4