Abstract
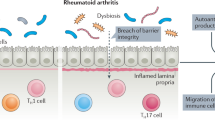
There is a growing understanding of the mechanisms by which the influence of the microbiota projects beyond sites of primary mucosal occupation to other human body systems. Bacteria present in the intestinal tract exert a profound effect on the host immune system, both locally and at distant sites. The oral cavity has its own characteristic microbiota, which concentrates in periodontal tissues and is in close association with a permeable epithelium. In this review we examine evidence which supports a role for the microbiome in the aetiology of rheumatic disease. We also discuss how changes in the composition of the microbiota, particularly within the gastrointestinal tract, may be affected by genetics, diet, and use of antimicrobial agents. Evidence is presented to support the theory that an altered microbiota is a factor in the initiation and perpetuation of inflammatory diseases, including rheumatoid arthritis (RA), spondyloarthritis (SpA), and inflammatory bowel disease (IBD). Mechanisms through which the microbiota may be involved in the pathogenesis of these diseases include altered epithelial and mucosal permeability, loss of immune tolerance to components of the indigenous microbiota, and trafficking of both activated immune cells and antigenic material to the joints. The potential to manipulate the microbiome, by application of probiotics and faecal microbial transplant (FMT), is now being investigated. Both approaches are in their infancy with regard to management of rheumatic disease but their potential is worthy of consideration, given the need for novel therapeutic approaches, and the emerging recognition of the importance of microbial interactions with human hosts.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Stebbings S, Munro K, Simon MA, Tannock G, Highton J, Harmsen H, et al. Comparison of the faecal microflora of patients with ankylosing spondylitis and controls using molecular methods of analysis. Rheumatol (Oxf). 2002;41(12):1395–401.
Bannatyne G, Wohlmann A. Rheumatoid Arthritis: its clinical history, etiology, and pathology. Lancet. 1896;147(3791):1120–5.
Human Microbiome Project C, Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, et al. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14.
Pineda MLA, Thompson SF, Summers K, de Leon F, Pope J, Reid G. A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Med Sci Monit. 2011;17(6):CR347–54.
Reveille JD. Genetic studies in the rheumatic diseases: present status and implications for the future. J Rheumatol Suppl. 2005;72:10–3.
Khan MA, Mathieu A, Sorrentino R, Akkoc N. The pathogenetic role of HLA-B27 and its subtypes. Autoimmun Rev. 2007;6(3):183–9.
Mathieu A, Paladini F, Vacca A, Cauli A, Fiorillo MT, Sorrentino R. The interplay between the geographic distribution of HLA-B27 alleles and their role in infectious and autoimmune diseases: a unifying hypothesis. Autoimmun Rev. 2009;8(5):420–5.
Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol. 2011;7(10):569–78.
Stebbings S, Jenks K, Roberts R, Schultz M. The immune response to gut bacteria in spondyloarthritis: a role in pathogenesis? JCRMM. 2010;1:1–10.
•• Ruutu M, Thomas G, Steck R, Degli-Esposti MA, Zinkernagel MS, Alexander K, et al. Beta-glucan triggers spondylarthritis and Crohn’s disease-like ileitis in SKG mice. Arthritis and Rheumatism. 2012;64(7):2211–22. The SKG mouse, a model for RA-like arthritis when derived in an SPF environment develops an SpA-like disease in response to microbial beta-glucans via a T H 17 cytokine pathway, a model suggesting microbial proteins may initiate SpA.
Simon GL, Gorbach SL. Intestinal flora in health and disease. Gastroenterology. 1984;86(1):174–93.
Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–33.
Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher HJ. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Micro. 2011;9(1):27–38.
Quigley EMM. Therapies aimed at the gut microbiota and inflammation: antibiotics, prebiotics, probiotics, synbiotics, anti-inflammatory therapies. Gastroenterol Clin North Am. 2011;40(1):207–22.
Loyola-Rodriguez JP, Martinez-Martinez RE, Abud-Mendoza C, Patiño-Marin N, Seymour GJ. Rheumatoid arthritis and the role of oral bacteria. J Oral Microbiol. 2010;2:1–8.
Dimmitt RA, Staley EM, Chuang G, Tanner SM, Soltau TD, Lorenz RG. Role of postnatal acquisition of the intestinal microbiome in the early development of immune function. J Pediatr Gastroenterol Nutr. 2010;51(3):262–73.
Isolauri E. Development of healthy gut microbiota early in life. J Paediatr Child Health. 2012;48 Suppl 3:1–6.
Urbaniak C, Burton JP, Reid G. Breast, milk and microbes: a complex relationship that does not end with lactation. Women’s Health (Lond Engl). 2012;8(4):385–98.
Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12(1):5–9.
Moore WEC, Moore LVH. The bacteria of periodontal diseases. Periodontology 2000. 1994;5(1):66–77.
Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183(12):3770–83.
Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontology 2000. 2006;42(1):80–7.
van Houte J. Role of micro-organisms in caries etiology. J Dent Res. 1994;73(3):672–81.
Löe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36(3):177–87.
Cullinan MP, Ford PJ, Seymour GJ. Periodontal disease and systemic health: current status. Aust Dent J. 2009;54:S62–9.
Gendron R, Grenier D, Maheu-Robert L-F. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect. 2000;2(8):897–906.
Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13(4):547–58.
Pizzo G, Guiglia R, Russo LL, Campisi G. Dentistry and internal medicine: from the focal infection theory to the periodontal medicine concept. Eur J Intern Med. 2010;21(6):496–502.
Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol (1970). 1999;70(7):793–802.
Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review. Ann Periodontol. 2003;8(1):38–53.
Seymour G, Ford P, Cullinan M, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13:3–10.
Abou-Raya A, Abou-Raya S, Abu-ElKheir H. Periodontal disease and rheumatoid arthritis: is there a link? Scand J Rheumatol. 2005;34(5):408–10.
Albandar JM. Some predictors of radiographic alveolar bone height reduction over 6 years. J Periodontal Res. 1990;25(3):186–92.
de Pablo P, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol. 2008;35(1):70–6.
Dissick A, Redman RS, Jones M, Rangan BV, Reimold A, Griffiths GR, et al. Association of periodontitis with rheumatoid arthritis: a pilot study. J Periodontol. 2010;81(2):223–30.
Käber UR, Michel A, Bolten WW, Gleissner C, Dehne F, Willershausen-Zönnchen B. Risk for periodontal disease in patients with longstanding rheumatoid arthritis. Arthritis Rheum. 1997;40(12):2248–51.
Mercado F, Marshall R, Klestov A, Bartold PM. Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 2001;72(6):779–87.
Jayatilake JAMS, Rajapakse S, Weerasinghe IE, Wanigasekara P, Vasanthathilaka J. Oral hygiene and periodontal status in a group of patients with rheumatoid arthritis. Indian J Rheumatol. 2011;6(3):111–5.
Al-Katma MK, Bissada NF, Bordeaux JM, Sue J, Askari AD. Control of periodontal infection reduces the severity of active rheumatoid arthritis. JCR: J Clin Rheumatol. 2007;13(3):134–7. doi:10.1097/RHU.0b013e3180690616.
Ortiz P, Bissada N, Palomo L, Han Y, Al-Zahrani M, Panneerselvam A, et al. Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumor necrosis factor inhibitors. J Periodontol. 2009;80(4):535–40.
Ribeiro J, Leão A, Novaes AB. Periodontal infection as a possible severity factor for rheumatoid arthritis. J Clin Periodontol. 2005;32(4):412–6.
Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33(6):401–7.
Kinane DF, Riggio MP, Walker KF, MacKenzie D, Shearer B. Bacteraemia following periodontal procedures. J Clin Periodontol. 2005;32(7):708–13.
Kempsell KE, Cox CJ, Hurle M, Wong A, Wilkie S, Zanders ED, et al. Reverse transcriptase-PCR analysis of bacterial rRNA for detection and characterization of bacterial species in arthritis synovial tissue. Infect Immun. 2000;68(10):6012–26.
Martinez-Martinez RE, Abud-Mendoza C, Patiño-Marin N, Rizo-Rodríguez JC, Little JW, Loyola-Rodríguez JP. Detection of periodontal bacterial DNA in serum and synovial fluid in refractory rheumatoid arthritis patients. J Clin Periodontol. 2009;36(12):1004–10.
Moen K, Brun J, Valen M, Skartveit L, Eribe E, Olsen I, et al. Synovial inflammation in active rheumatoid arthritis and psoriatic arthritis facilitates trapping of a variety of oral bacterial DNAs. Clin Exp Rheumatol. 2006;24(6):656–63.
Moen K, Brun JG, Eribe ERK, Olsen I, Jonsson R. Oral bacterial DNAs in synovial fluids of arthritis patients. Microb Ecol Heal Dis. 2005;17(1):2–8.
Témoin S. Identification of oral bacterial DNA in synovial fluid of patients with arthritis with native and failed prosthetic joints. J Clin Rheumatol. 2012;18(3):117.
Wegner N, Wait R, Sroka A, Eick S, Nguyen K-A, Lundberg K, et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010;62(9):2662–72.
Haffajee AD, Bogren A, Hasturk H, Feres M, Lopez NJ, Socransky SS. Subgingival microbiota of chronic periodontitis subjects from different geographic locations. J Clin Periodontol. 2004;31(11):996–1002.
Nesse W, Westra J, van der Wal JE, Abbas F, Nicholas AP, Vissink A, et al. The periodontium of periodontitis patients contains citrullinated proteins which may play a role in ACPA (anti-citrullinated protein antibody) formation. J Clin Periodontol. 2012;39(7):599–607.
Klareskog L, Rönnelid J, Lundberg K, Padyukov L, Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu Rev Immunol. 2008;26(1):651–75.
Masson-Bessière C. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the α-and β-chains of fibrin. J Immunol (1950). 2001;166(6):4177.
Routsias JG. Autopathogenic correlation of periodontitis and rheumatoid arthritis. Rheumatol (Oxf, Engl). 2011;50(7):1189–93.
Vossenaar ER. Citrullinated proteins: sparks that may ignite the fire in rheumatoid arthritis. Arthritis Res Ther. 2004;6(3):107.
Mikuls TR, Payne JB, Reinhardt RA, Thiele GM, Maziarz E, Cannella AC, et al. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int Immunopharmacol. 2009;9(1):38–42.
Lundberg K, Kinloch A, Fisher BA, Wegner N, Wait R, Charles P, et al. Antibodies to citrullinated α-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008;58(10):3009–19.
Lundberg K, Wegner N, Yucel-Lindberg T, Venables PJ. Periodontitis in RA-the citrullinated enolase connection. Nat Rev Rheumatol. 2010;6(12):727–30.
Hitchon CA, Chandad F, Ferucci ED, Willemze A, Ioan-Facsinay A, Van der Woude D, et al. Antibodies to Porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J Rheumatol. 2010;37(6):1105–12.
Askling J, Grahnquist L, Ekbom A, Finkel Y. Incidence of paediatric Crohn’s disease in Stockholm, Sweden. Lancet. 1999;354(9185):1179.
Turunen P, Kolho KL, Auvinen A, Iltanen S, Huhtala H, Ashorn M. Incidence of inflammatory bowel disease in Finnish children, 1987–2003. Inflamm Bowel Dis. 2006;12(8):677–83.
Gent AE, Hellier MD, Grace RH, Swarbrick ET, Coggon D. Inflammatory bowel disease and domestic hygiene in infancy. Lancet. 1994;343(8900):766–7.
Hammer RE, Maika SD, Richardson JA, Tang JP, Taurog JD. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2 m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63(5):1099–112.
Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernández-Sueiro JL, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180(6):2359–64.
Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm Jr TE, Balish E, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98(4):945–53.
Sakaguchi S, Takahashi T, Hata H, Nomura T, Sakaguchi N. SKG mice, a new genetic model of rheumatoid arthritis. Arthritis Res Ther. 2003;5 Suppl 3:10.
Gibson G. Growth and activities of sulphate-reducing bacteria in gut contents of healthy subjects and patients with ulcerative colitis. FEMS Microbiol Lett. 1991;86(2):103–11.
Matsuda H, Fujiyama Y, Andoh A, Ushijima T, Kajinami T, Bamba T. Characterization of antibody responses against rectal mucosa-associated bacterial flora in patients with ulcerative colitis. J Gastroenterol Hepatol. 2000;15(1):61–8.
Carter JD. Bacterial agents in spondyloarthritis: a destiny from diversity? Best Pract Res Clin Rheumatol. 2010;24(5):701–14.
Schwimmbeck PL, Yu DT, Oldstone MB. Autoantibodies to HLA B27 in the sera of HLA B27 patients with ankylosing spondylitis and Reiter’s syndrome. Molecular mimicry with Klebsiella pneumoniae as potential mechanism of autoimmune disease. J Exp Med. 1987;166(1):173–81.
Lopez de Castro J. HLA-B27 and the pathogenesis of spondyloarthropathies. Immunol Lett. 2007;108(1):27–33.
Edwards CJ. Commensal gut bacteria and the etiopathogenesis of rheumatoid arthritis. J Rheumatol. 2008;35(8):1477–14797.
Vaahtovuo J, Munukka E, Korkeamaki M, Luukkainen R, Toivanen P. Fecal microbiota in early rheumatoid arthritis. J Rheumatol. 2008;35(8):1500–5.
Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, et al. Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut. 2003;52(2):237–42.
Korzenik JR. Past and current theories of etiology of IBD: toothpaste, worms, and refrigerators. J Clin Gastroenterol. 2005;39(4 Suppl 2):S59–65.
Angelakis E, Bastelica D, Ben Amara A, El Filali A, Dutour A, Mege JL, et al. An evaluation of the effects of Lactobacillus ingluviei on body weight, the intestinal microbiome and metabolism in mice. Microb Pathog. 2012;52(1):61–8.
Elli M, Colombo O, Tagliabue A. A common core microbiota between obese individuals and their lean relatives? Evaluation of the predisposition to obesity on the basis of the fecal microflora profile. Med Hypotheses. 2010;75(4):350–2.
Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, Kitas GD. Obesity in rheumatoid arthritis. Rheumatol (Oxf, Engl). 2011;50(3):450–62.
Callahan LF, Mielenz T, Freburger J, Shreffler J, Hootman J, Brady T, et al. A randomized controlled trial of the people with arthritis can exercise program: symptoms, function, physical activity, and psychosocial outcomes. Arthritis Rheum. 2008;59(1):92–101.
Giles JT, Ling SM, Ferrucci L, Bartlett SJ, Andersen RE, Towns M, et al. Abnormal body composition phenotypes in older rheumatoid arthritis patients: association with disease characteristics and pharmacotherapies. Arthritis Rheum. 2008;59(6):807–15.
Cani PD, Delzenne NM. Involvement of the gut microbiota in the development of low grade inflammation associated with obesity: focus on this neglected partner. Acta Gastro-Enterol Belg. 2010;73(2):267–9.
Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50(1):90–7.
Scotece M, Conde J, Gómez R, López V, Pino J, González A, et al. Role of adipokines in atherosclerosis: interferences with cardiovascular complications in rheumatic diseases. Mediat Inflamm. 2012;2012:1–14.
Senolt L, Krystufkova O, Hulejova H, Kuklova M, Filkova M, Cerezo LA, et al. The level of serum visfatin (PBEF) is associated with total number of B cells in patients with rheumatoid arthritis and decreases following B cell depletion therapy. Cytokine. 2011;55(1):116–21.
Busso N, Karababa M, Nobile M, Rolaz A, Van Gool F, Galli M, et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS One. 2008;3(5):e2267.
Rho YH, Solus J, Sokka T, Oeser A, Chung CP, Gebretsadik T, et al. Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis. Arthritis Rheum. 2009;60(7):1906–14.
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81.
Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–103.
Muller H, de Toledo FW, Resch KL. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: a systematic review. Scand J Rheumatol. 2001;30(1):1–10.
Antonopoulos DA, Huse SM, Morrison HG, Schmidt TM, Sogin ML, Young VB. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect Immun. 2009;77(6):2367–75.
De La Cochetiere MF, Durand T, Lepage P, Bourreille A, Galmiche JP, Dore J. Resilience of the dominant human fecal microbiota upon short-course antibiotic challenge. J Clin Microbiol. 2005;43(11):5588–92.
Nieuwenhuis EE, Visser MR, Kavelaars A, Cobelens PM, Fleer A, Harmsen W, et al. Oral antibiotics as a novel therapy for arthritis: evidence for a beneficial effect of intestinal Escherichia coli. Arthritis Rheum. 2000;43(11):2583–9.
O’Dell JR, Paulsen G, Haire CE, Blakely K, Palmer W, Wees S, et al. Treatment of early seropositive rheumatoid arthritis with minocycline: four-year followup of a double-blind, placebo-controlled trial. Arthritis Rheum. 1999;42(8):1691–5.
O’Dell JR, Blakely KW, Mallek JA, Eckhoff PJ, Leff RD, Wees SJ, et al. Treatment of early seropositive rheumatoid arthritis: a two-year, double-blind comparison of minocycline and hydroxychloroquine. Arthritis Rheum. 2001;44(10):2235–41.
Hannonen P, Mottonen T, Hakola M, Oka M. Sulfasalazine in early rheumatoid arthritis. A 48-week double-blind, prospective, placebo-controlled study. Arthritis Rheum. 1993;36(11):1501–9.
Weinblatt ME, Reda D, Henderson W, Giobbie-Hurder A, Williams D, Diani A, et al. Sulfasalazine treatment for rheumatoid arthritis: a metaanalysis of 15 randomized trials. J Rheumatol. 1999;26(10):2123–30.
Chen J, Liu C. Is sulfasalazine effective in ankylosing spondylitis? A systematic review of randomized controlled trials. J Rheumatol. 2006;33(4):722–31.
Mikov M, Lee HJ, Fawcett JP. The influence of probiotic treatment on sulfasalazine metabolism in rat gut contents. Asian J Pharmacodynam Pharmacokinet. 2006;6(4):337–42.
• Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4554–61. This study examines the effect of a short course of ciprofloxacin on the human microbiota. It demonstrates that profound changes in the ecology of the gut can occur with antibiotics within a few days, and this may result in an altered stable flora. The consequences of this remain unknown.
Morris AJ, Howden CW, Robertson C, Duncan A, Torley H, Sturrock RD, et al. Increased intestinal permeability in ankylosing spondylitis—primary lesion or drug effect? Gut. 1991;32(12):1470–2.
Martinez-Gonzalez O, Cantero-Hinojosa J, Paule-Sastre P, Gomez-Magan JC, Salvatierra-Rios D. Intestinal permeability in patients with ankylosing spondylitis and their healthy relatives. Br J Rheumatol. 1994;33(7):644–7.
Bjarnason I, Takeuchi K. Intestinal permeability in the pathogenesis of NSAID-induced enteropathy. J Gastroenterol. 2009;44 Suppl 19:23–9.
Mielants H, Veys EM, De Vos M, Cuvelier C. Increased intestinal permeability in ankylosing spondylitis. Gut. 1992;33(8):1150–50.
Gilbert RS, Kobayashi R, Sekine S, Fujihashi K. Functional transforming growth factor-beta receptor type II expression by CD4+ T cells in Peyer’s patches is essential for oral tolerance induction. PLoS One. 2011;6(11):e27501.
• Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34(2):237–46. The authors used several mouse models to show that T REG cells, after generation in lymph nodes, move to the gut and undergo local expansion, developing oral tolerance, a process involving IL-10 production.
Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30(1):80–9.
Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8(12):1380–9.
Stebbings SM, Taylor C, Tannock GW, Baird MA, Highton J. The immune response to autologous bacteroides in ankylosing spondylitis is characterized by reduced interleukin 10 production. J Rheumatol. 2009;36(4):797–800.
Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol. 1995;102(3):448–55.
Blaschke S, Middel P, Dorner BG, Blaschke V, Hummel KM, Kroczek RA, et al. Expression of activation-induced, T cell-derived, and chemokine-related cytokine/lymphotactin and its functional role in rheumatoid arthritis. Arthritis Rheum. 2003;48(7):1858–72.
May E, Märker-Hermann E, Wittig BM, Zeitz M, Büschenfelde KMZ, Duchmann R. Identical T-cell expansions in the colon mucosa and the synovium of a patient with enterogenic spondyloarthropathy. Gastroenterology. 2000;119(6):1745–55.
Salmi M, Jalkanen S. Human leukocyte subpopulations from inflamed gut bind to joint vasculature using distinct sets of adhesion molecules. J Immunol. 2001;166(7):4650–7.
Sheldon P. Rheumatoid arthritis and gut related lymphocytes: the iteropathy concept. Ann Rheum Dis. 1988;47(8):697–700.
FAO/WHO. Probiotics in food. Health and nutritional properties and guidelines for evaluation. FAO Food and Nutrition Paper No. 85 (ISBN 92-5-105513-0) 2006.
Salazar N, Binetti A, Gueimonde M, Alonso A, Garrido P, del Gonzalez RC, et al. Safety and intestinal microbiota modulation by the exopolysaccharide-producing strains Bifidobacterium animalis IPLA R1 and Bifidobacterium longum IPLA E44 orally administered to Wistar rats. Int J Food Microbiol. 2011;144(3):342–51.
McNulty NP, Yatsunenko T, Hsiao A, Faith JJ, Muegge BD, Goodman AL, et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med. 2011;3(106):106ra06.
Amdekar S, Singh V, Singh R, Sharma P, Keshav P, Kumar A. Lactobacillus casei reduces the inflammatory joint damage associated with collagen-induced arthritis (CIA) by reducing the pro-inflammatory cytokines: Lactobacillus casei: COX-2 inhibitor. J Clin Immunol. 2011;31(2):147–54.
So JS, Kwon HK, Lee CG, Yi HJ, Park JA, Lim SY, et al. Lactobacillus casei suppresses experimental arthritis by down-regulating T helper 1 effector functions. Mol Immunol. 2008;45(9):2690–9.
Jenks K, Stebbings S, Burton J, Schultz M, Herbison P, Highton J. Probiotic therapy for the treatment of spondyloarthritis: a randomized controlled trial. J Rheumatol. 2010;37(10):2118–25.
Teughels W, Loozen G, Quirynen M. Do probiotics offer opportunities to manipulate the periodontal oral microbiota? J Clin Periodontol. 2011;38 Suppl 11:159–77.
De Keyser F, Elewaut D, De Vos M, De Vlam K, Cuvelier C, Mielants H, et al. Bowel inflammation and the spondyloarthropathies. Rheum Dis Clin North Am. 1998;24(4):785–813. ix–x.
Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44(5):854–9.
Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37(1):42.
Allen-Vercoe E, Reid G, Viner N, Gloor GB, Hota S, Kim P, et al. A Canadian Working Group report on fecal microbial therapy: microbial ecosystems therapeutics. Can J Gastroenterol. 2012;26(7):457–62.
Garborg K, Waagsbo B, Stallemo A, Matre J, Sundoy A. Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis. 2010;42(11–12):857–61.
Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(5):761–7.
Kleessen B, Kroesen A, Buhr H, Blaut M. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroentero. 2002;37(9):1034–41.
Ebringer R, Cooke D, Cawdell DR, Cowling P, Ebringer A. Ankylosing spondylitis: klebsiella and HL-A B27. Rheumatol Rehabil. 1977;16(3):190–6.
Ebringer RW, Cawdell DR, Cowling P, Ebringer A. Sequential studies in ankylosing spondylitis. Association of Klebsiella pneumoniae with active disease. Ann Rheum Dis. 1978;37(2):146–51.
Shinebaum R, Neumann VC, Cooke EM, Wright V. Comparison of faecal florae in patients with rheumatoid arthritis and controls. Brit J Rheumatol. 1987;26(5):329–33.
Aoki S, Yoshikawa K, Yokoyama T, Nonogaki T, Iwasaki S, Mitsui T, et al. Role of enteric bacteria in the pathogenesis of rheumatoid arthritis: evidence for antibodies to enterobacterial common antigens in rheumatoid sera and synovial fluids. Ann Rheum Dis. 1996;55(6):363–9.
Kanazawa K, Haga Y, Funakoshi O, Nakajima H, Munakata A, Yoshida Y. Absence of Mycobacterium paratuberculosis DNA in intestinal tissues from Crohn’s disease by nested polymerase chain reaction. J Gastroenterol 1999;34(2):200–6.
Kreuzpaintner G, Das PK, Stronkhorst A, Slob AW, Strohmeyer G. Effect of intestinal resection on serum antibodies to the mycobacterial 45/48 kilodalton doublet antigen in Crohn's disease. Gut 1995;37(3):361–6.
Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Folsch UR, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 2004;53(5):685–93.
Zhang L, Day A, McKenzie G, Mitchell H. Nongastric Helicobacter species detected in the intestinal tract of children. J Clin Microbiol. 2006;44(6):2276–9.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Rheumatoid Arthritis
Rights and permissions
About this article
Cite this article
Yeoh, N., Burton, J.P., Suppiah, P. et al. The Role of the Microbiome in Rheumatic Diseases. Curr Rheumatol Rep 15, 314 (2013). https://doi.org/10.1007/s11926-012-0314-y
Published:
DOI: https://doi.org/10.1007/s11926-012-0314-y