Abstract
Purpose
Adverse symptom event reporting is vital as part of clinical trials and drug labeling to ensure patient safety and inform risk–benefit decision making. The purpose of this study was to assess the reliability of adverse event reporting of different clinicians for the same patient for the same visit.
Methods
A retrospective reliability analysis was completed for a sample of 393 cancer patients (42.8% men; age 26–91, M = 62.39) from lung (n = 134), prostate (n = 113), and Ob/Gyn (n = 146) clinics. These patients were each seen by two clinicians who independently rated seven Common Terminology Criteria for Adverse Events (CTCAE) symptoms. Twenty-three percent of patients were enrolled in therapeutic clinical trials.
Results
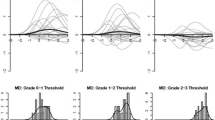
The average time between rater evaluations was 68 min. Intraclass correlation coefficients were moderate for constipation (0.50), diarrhea (0.58), dyspnea (0.69), fatigue (0.50), nausea (0.52), neuropathy (0.71), and vomiting (0.46). These values demonstrated stability over follow-up visits. Two-point differences, which would likely affect treatment decisions, were most frequently seen among symptomatic patients for constipation (18%), vomiting (15%), and nausea (8%).
Conclusion
Agreement between different clinicians when reporting adverse symptom events is moderate at best. Modification of approaches to adverse symptom reporting, such as patient self-reporting, should be considered.

Similar content being viewed by others
Notes
Additional information on the PRO-CTCAE initiative can be found at https://wiki.nci.nih.gov/x/cKul
Abbreviations
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- ICC(s):
-
Intraclass correlation coefficient(s)
- MedDRA:
-
Medical dictionary of regulatory activities
- MRN(s):
-
Medical record number(s)
- NCI:
-
National Cancer Institute
- PRO(s):
-
Patient-reported outcome(s)
- PRO-CTCAE:
-
Patient-reported outcomes version of the Common Terminology Criteria for Adverse Events
References
NCI: National Cancer Institute. (2001). Cancer therapy evaluation program. NCI guidelines—Expedited adverse event reporting requirements for NCI investigational agents. Bethesda: National Cancer Institute.
Basch, E., Iasonos, A., Barz, A., et al. (2007). Long-term toxicity monitoring via electronic patient-reported outcomes in patients receiving chemotherapy. Journal of Clinical Oncology, 25, 5374–5380.
Basch, E. (2010). The missing voice of patients in drug-safety reporting. New England Journal of Medicine, 362, 865–869.
Basch, E., Jia, X., Heller, G., et al. (2009). Adverse symptom event reporting by patients vs clinicians: Relationships with clinical outcomes. Journal of the National Cancer Institute, 101, 1624–1632.
Belknap, S. M., Georgopoulos, C. H., West, D. P., et al. (2010). Quality of methods for assessing and reporting serious adverse events in clinical trials of cancer drugs. Clinical Pharmacology and Therapeutics, 88, 231–236.
Ahmad, S. R. (2003). Adverse drug event monitoring at the Food and Drug Administration. Journal of General Internal Medicine, 18, 57–60.
Trotti, A., Colevas, A. D., Setser, A., et al. (2007). Patient-reported outcomes and the evolution of adverse event reporting in oncology. Journal of Clinical Oncology, 25, 5121–5127.
Trotti, A., Colevas, A. D., Setser, A., et al. (2003). CTCAE v3.0: Development of a comprehensive grading system for the adverse events of cancer treatment. Seminars in Radiation Oncology, 13, 176–181.
Basch, E., Iasonos, A., McDonough, T., et al. (2006). Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: Results of a questionnaire-based study. The Lancet Oncology, 7, 903–909.
Bruner, D. W., Bryan, C. J., Aaronson, N., et al. (2007). Issues and challenges with integrating patient-reported outcomes in clinical trials supported by the National Cancer Institute-sponsored clinical trials networks. Journal of Clinical Oncology, 25, 5051–5057.
US Food and Drug Administration. (2009). Guidance for industry. Patient-reported outcome measures: Use in medical development to support labeling claims. Available from http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. Accessed 9 Dec 2010
Uebersax, J. S. (1987). Diversity of decision-making models and the measurement of interrater agreement. Psychological Bulletin, 101, 140–146.
Shrout, P. E., & Fleiss, J. L. (1979). Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin, 86, 420–428.
Rosner, B. (2005). Fundamentals of biostatistics. Belmont, CA: Duxbury Press.
McGill, R., Tukey, J. W., & Larsen, W. A. (1978). Variation of box plots. The American Statistician, 32, 12–16.
Zegers, M., de Bruijne, M. C., Wagner, C., et al. (2010). The inter-rater agreement of retrospective assessments of adverse events does not improve with two reviewers per patient record. Journal of Clinical Epidemiology, 63, 94–102.
Weingart, S. N., Gandhi, T. K., Seger, A. C., et al. (2005). Patient-reported medication symptoms in primary care. Archives of Internal Medicine, 165, 234–240.
Berry, D. L., Moinpour, C. M., Jiang, C. S., et al. (2006). Quality of life and pain in advanced stage prostate cancer: Results of a Southwest Oncology Group randomized trial comparing docetaxel and estramustine to mitoxantrone and prednisone. Journal of Clinical Oncology, 24, 2828–2835.
Pakhomov, S., Jacobsen, S. J., Chute, C. G., et al. (2008). Agreement between patient-reported symptoms and their documentation in the medical record. The American Journal of Managed Care, 14, 530–539.
Hahn, E. A., Cella, D., Chassany, O., et al. (2007). Precision of health-related quality-of-life data compared with other clinical measures. Mayo Clinic Proceedings, 82, 1244–1254.
Ioannidis, J. P., Evans, S. J., Gøtzsche, P. C., et al. (2004). Better reporting of harms in randomized trials: An extension of the CONSORT statement. Annals of Internal Medicine, 141, 781–788.
Institute of Medicine, National Academy of Sciences. (2006). The future of drug safety: Promoting and protecting the health of the public. Washington, DC: National Academies Press.
Kirkova, J., Davis, M. P., Walsh, D., et al. (2006). Cancer symptom assessment instruments: A systematic review. Journal of Clinical Oncology, 24, 1459–1473.
Acknowledgments
This project was supported by a National Institutes of Health Research Training Grant (T32 CA009461-25); a National Institutes of Health Support Grant (P30-CA-008748). The findings in this manuscript were partially reported at the 31st Annual Meeting and Scientific Sessions of the Society of Behavioral Medicine, Seattle, WA, April 7–10, 2010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atkinson, T.M., Li, Y., Coffey, C.W. et al. Reliability of adverse symptom event reporting by clinicians. Qual Life Res 21, 1159–1164 (2012). https://doi.org/10.1007/s11136-011-0031-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-011-0031-4