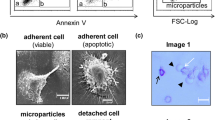
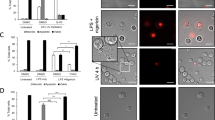
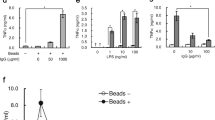
Microparticles are small membrane vesicles released from the cell membrane by exogenous budding. To elucidate the interactions of microparticles with macrophages, the effect of microparticles released from Jurkat T cells on RAW 264.7 cells was determined. Microparticles were isolated by differential centrifugation, using FACS analysis with annexin V and cell surface markers for identification. Various inducers of apoptosis increased the release of microparticles from Jurkat cells up to 5-fold. The released microparticles were then cultured with RAW 264.7 cells. As shown by confocal microscopy and FACS analysis, RAW 264.7 macrophages cleared microparticles by phagocytosis. In addition, microparticles induced apoptosis in RAW 264.7 cells in a dose-dependent manner with up to a 5-fold increase of annexin V positive cells and 9-fold increase in caspase 3 activity. Cell proliferation as determined by the MTT test was also reduced. Furthermore, microparticles stimulated the release of microparticles from macrophages. These effects were specific for macrophages, since no apoptosis was observed in NIH 3T3 and L929 cells. These findings indicate that microparticles can induce macrophages to undergo apoptosis, in turn resulting in a further increase of microparticles. The release of microparticles from apoptotic cells may therefore represent a novel amplification loop of cell death.
Similar content being viewed by others
References
Savill J, Haslett C. Granulocyte clearance by apoptosis in the resolution of inflammation. Semin Cell Biol 1995; 6: 385–393.
Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 1998; 101: 890–898.
Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest 2002; 109: 41–50.
Stuart LM, Lucas M, Simpson C, Lamb J, Savill J, Lacy-Hulbert A. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol 2002; 168: 1627–1635.
Urban BC, Willcox N, Roberts DJ. A role for CD36 in the regulation of dendritic cell function. Proc Natl Acad Sci USA 2001; 98: 8750–8755.
Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, and Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature 1997; 390: 350–351.
Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002; 418: 191–195.
Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature 2000; 407: 784–788.
Savill J. Recognition and phagocytosis of cells undergoing apoptosis. Br Med Bull 1997; 53: 491–508.
Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2002; 2: 965–975.
Platt N, da Silva RP, Gordon S. Recognizing death: The phagocytosis of apoptotic cells. Trends Cell Biol 1998; 8: 365–372.
Savill J. Apoptosis in resolution of inflammation. Kidney Blood Press Res 2000; 23: 173–174.
Aupeix K, Hugel B, Martin T, et al. The significance of shed membrane particles during programmed cell death in vitro, and in vivo, in HIV-1 infection. J Clin Invest 1997; 99: 1546–1554.
Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, et al. Proteomic analysis of dendritic cell-derived exosomes: A secreted subcellular compartment distinct from apoptotic vesicles. J Immunol 2001; 166: 7309–7318.
Mesri M, Altieri DC. Endothelial cell activation by leukocyte microparticles. J Immunol 1998; 161: 4382–4387.
Mesri M, Altieri DC. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J Biol Chem 1999; 274: 23111–23118.
Combes V, Simon AC, Grau GE, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest 1999; 104: 93–102.
Gilbert GE, Sims PJ, Wiedmer T, Furie B, Furie BC, Shattil SJ. Platelet-derived microparticles express high affinity receptors for factor VIII. J Biol Chem 1991; 266: 17261–17268.
Berckmans RJ, Neiuwland R, Boing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost 2001; 85: 639–646.
Falati S, Liu Q, Gross P, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med 2003; 197: 1585–1598.
Knijff-Dutmer EA, Koerts J, Nieuwland R, Kalsbeek-Batenburg EM, van de Laar MA. Elevated levels of platelet microparticles are associated with disease activity in rheumatoid arthritis. Arthritis Rheum 2002; 46: 1498–1503.
Minagar A, Jy W, Jimenez JJ, et al. Elevated plasma endothelial microparticles in multiple sclerosis. Neurology 2001; 56: 1319–1324.
Zhang J, Driscoll TA, Hannun YA, Obeid LM. Regulation of membrane release in apoptosis. Biochem J 1998; 334(Pt 2): 479–485.
Riley PA, Dean RT. Phagocytosis of latex particles in relation to the cell cycle in 3T3 cells. Exp Cell Biol 1978; 46: 367–373.
Kim JS, Kwon HY, Choi WH, et al. Phagocytosis of serum- and IgG-opsonized zymosan particles induces apoptosis through superoxide but not nitric oxide in macrophage J774A.1. Exp Mol Med 2003; 35: 211–221.
Evans SM, Ashwood P, Warley A, Berisha F, Thompson RP, Powell JJ. The role of dietary microparticles and calcium in apoptosis and interleukin-1beta release of intestinal macrophages. Gastroenterology 2002; 123: 1543–1553.
Yuan XM. Apoptotic macrophage-derived foam cells of human atheromas are rich in iron and ferritin, suggesting iron-catalysed reactions to be involved in apoptosis. Free Radic Res 1999; 30: 221–231.
Hiura TS, Kaszubowski MP, Li N, Nel AE. Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages. J Immunol 1999; 163: 5582–5591.
Jiang N, Reich CF III, Pisetsky DS. Role of macrophages in the generation of circulation blood nucleosomes from dead and dying cells. Blood 2003; 102: 2243–2250.
Jeha S. Tumor lysis syndrome. Semin Hematol 2001; 38: 4–8.
Altman A. Acute tumor lysis syndrome. Semin Oncol 2001; 28: 3–8.
Savill J. Apoptosis. Phagocytic docking without shocking. Nature 1998; 392: 442–443.
Taylor PR, Carugati A, Fadok VA, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med 2000; 192: 359–366.
Botto M, Dell’Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet 1998; 19: 56–59.
Berckmans RJ, Nieuwland R, Tak PP, et al. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum 2002; 46: 2857–2866.
Brogan PA, Shah V, Brachet C, et al. Endothelial and platelet microparticles in vasculitis of the young. Arthritis Rheum 2004; 50: 927–936.
Distler JHW, Jüngel A, Huber LC, et al. The Induction of Matrix Metalloproteinase and Cytokine Expression in Synovial Fibroblasts Stimulated with Immune Cell Microparticles. Proc Natl Acad Sci USA 2005; 162(8): 2852–2857.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Distler, J.H.W., Huber, L.C., Hueber, A.J. et al. The release of microparticles by apoptotic cells and their effects on macrophages. Apoptosis 10, 731–741 (2005). https://doi.org/10.1007/s10495-005-2941-5
Issue Date:
DOI: https://doi.org/10.1007/s10495-005-2941-5