Abstract
As part of the study “The Burden of Rheumatoid Arthritis (RA) and Patient Access to Treatment”, this paper reviews the impact on access to RA drugs of the approval processes, pricing and funding decisions and times to market (access) in different countries. In addition, an overview of health technology assessments (HTA) and the economic literature related to RA treatments is provided. The time from approval to market access ranged from immediate to over 500 days in the countries included in the study. A total of 55 HTA reports were identified, 40 of them in the period between 2002 and 2006; 29 were performed by European HTA agencies, 14 in Canada and 7 in the United States. A total of 239 economic evaluations related to RA were identified in a specialized health economic database (HEED).




Similar content being viewed by others
References
Magnusson, S.: Treatment of rheumatoid arthritis—does it affect society’s cost for the disease? Br. J. Rheumatol. 35, 791–795 (1996)
EFPIA (2002) The European Federation of Pharmaceutical Industries and Associations annual report. http://www.efpia.org/6_publ/annual/Report2002.pdf
DiMasi, J.A., Hansen, R.W., Grabowski, H.G., Lasagna, L.: Cost of innovation in the pharmaceutical industry. J. Health Econ. 10, 107–142 (1991)
DiMasi, J.A., Hansen, R.W., Grabowski, H.G.: The price of innovation: new estimates of drug development costs. J. Health Econ. 22, 151–185 (2003)
Adams, C.P., Brantner, V.V.: Estimating the cost of new drug development: is it really 802 million dollars? Health Aff (Millwood) 25, 420–428 (2006)
Motola, D., De Ponti, F., Poluzzi, E., et al.: An update on the first decade of the European centralized procedure: how many innovative drugs? Br. J. Clin. Pharmacol. 62, 610–616 (2006)
European Medicines Agency (EMEA). http://www.emea.eu.int
Anderson, C., McAuslane, N., Walker, S.: The impact of the changing regulatory environment on review times. CMR, International R&D briefing No.35 (online). http://www.cmr.org/pdf/r_d35.pdf (2002)
Health Canada (2005) Priority review of drug submissions. Guidance Date 30 11 2005
Food and Drug Administration (FDA) USA. http://www.fda.gov
Danzon, P.M., Furukawa, M.F.: Prices and availability of pharmaceuticals: evidence from nine countries. Health Aff (Millwood) (Suppl) Web Exclus. W3:521–536 (2003)
Pharmaceutical Price Controls in OECD Countries (2004) Implications of U.S. Consumers, Pricing, Research and Development, and Innovation. U.S. Department of Commerce International Trade Administration, Washington, DC
Patients W.A.I.T. Indicator (2007) Phase 6 Report. IMS Management Consulting
National Institute for Clinical Excellence (2002) Guidance on the use of etanercept and infliximab for the treatment of rheumatoid arthritis. Technol. Appraisal no. 36 (March)
Sundhedsstyrelsen Center for Evaluering og Medicinsk Teknologivurdering. Rheumatoid Arthritis (2002) Health Technology Assessment of diagnosis and treatment. Medicinsk Teknologivurdering 4
Coyle, D., Judd, M., Blumenauer, B., et al.: Infliximab and etanercept in patients with rheumatoid arthritis: a systematic review and economic evaluation (Technology report no 64). Canadian Coordinating Office for Health Technology Assessment, Ottawa (2006)
National Institute for Clinical Excellence (2003) Anakinra for rheumatoid arthritis. Technol. Appraisal 72 (November)
Conflict of interest
This study has been funded by unrestricted grant from F. Hoffmann-La Roche Ltd., Basel, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Additional information
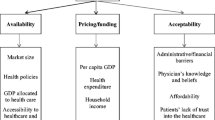
This section deals with access to rheumatoid arthritis (RA) drugs. There are a number of steps, and determinants influencing these steps, before a drug can be prescribed to and used by a patient, including research and development, regulatory approval process, pricing, reimbursement, and adoption by prescribes.
Rights and permissions
About this article
Cite this article
Lundqvist, J., Kastäng, F., Kobelt, G. et al. The burden of rheumatoid arthritis and access to treatment: determinants of access. Eur J Health Econ 8 (Suppl 2), 87–93 (2008). https://doi.org/10.1007/s10198-007-0090-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-007-0090-1