Abstract
Background
Leukocyte adhesion molecules are important for migration of the inflammatory cells into sites of inflammation. Intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) are members of the immunoglobulin superfamily that are expressed in normal kidney. Their expression is up-regulated in the renal tissue of patients with lupus nephritis (LN).
Objectives
We evaluated whether changes in urinary levels of ICAM-1 and VCAM-1 reflect renal tissue damage in LN. We related the levels of these molecules to other laboratory findings, especially complement C3/C4 levels. We also tested the hypothesis that changes in urinary levels of ICAM-1 and VCAM-1 reflect the severity of renal tissue damage in LN.
Patients and methods
This study included 30 systemic lupus erythematosus (SLE) patients with LN (16 with mild histological changes, i.e., with World Health Organization (WHO) class I and II LN, and 14 with advanced histological changes, i.e., class III, IV, and V LN) and 20 with SLE without nephritis. In addition, 20 healthy individuals of comparable age were included as a control group. The levels of urinary ICAM-1 and VCAM-1 were measured by enzyme-linked immunosorbent assay (ELISA) and related to the clinical, laboratory [rheumatoid factor(RF), antinuclear antibodies (ANA), anti-double-stranded DNA (anti-dsDNA), complements C3 and C4] and histological findings.
Results
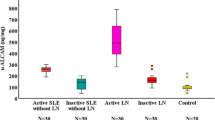
Levels of urinary ICAM-1 and VCAM-l in LN patients with advanced histological changes (renal damage) were statistically significantly higher than those in other groups (LN patients with mild histological changes or SLE patients without nephritis and control group; p < 0.01). In contrast, serum levels of C3 and C4 in LN patients with advanced histological changes were significantly lower than those in other groups (p < 0.01). There was a significant negative correlation between the levels of urinary adhesion molecules and serum complement levels (p < 0.01).
Conclusions
The significantly high urinary levels of the adhesion molecules in the LN group with advanced histological changes may reflect their renal tissue expression and therefore the severity of the nephritis. Renal tissue damage in these cases may be the result of transmigration of activated inflammatory cells, inducing serious tissue damage. The hypocomplementemia combined with increased urinary levels of adhesion molecules seems to be a useful biomarker of disease severity in LN.





Similar content being viewed by others
References
Springer TA. Adhesion receptors of the immune system. Nature. 1990;346(6283):425–34.
Brady HR. Leukocyte adhesion molecules and kidney diseases. Kidney Int. 1994;45(5):1285–300.
Norman MU, James WG, Hickey MJ. Differential roles of ICAM-1 and VCAM-1 in leukocyte-endothelial cell interactions in skin and brain of MRL/faslpr mice. J Leukoc Biol. 2008;84(1):68–76.
Lhotta K, Neumayer HP, Joannidis M, Geissler D, Konig P. Renal expression of intercellular adhesion molecule-1 in different forms of glomerulonephritis. Clin Sci (Lond). 1991;81(4):477–81.
Chen X, Xu Q, Tang L. Expression of intercellular adhesion molecule-1 and vascular adhesion molecule-1 in kidney of patients with lupus nephritis and membranoproliferative glomerulonephritis. Zhonghua Bing Li Xue Za Zhi. 1995;24(3):149–51.
Zheng L, Sinniah R, Hsu SI. In situ glomerular expression of activated NF-kappaB in human lupus nephritis and other non-proliferative proteinuric glomerulopathy. Virchows Arch. 2006;448(2):172–83.
Yokoyama H, Takaeda M, Wada T, Ohta S, Hisada Y, Segawa C, Furuichi K, Kobayashi K. Glomerular ICAM-1 expression related to circulating TNF-alpha in human glomerulonephritis. Nephron. 1997;76(4):425–33.
Ma L, Zou W, Zhang Z. In situ expression of intercellular adhesion molecule-1 in human glomerulonephritis. Zhonghua Yi Xue Za Zhi. 1995;75(4):201–3, 253 (Article in Chinese).
Rabb H, O’Meara YM, Maderna P, Coleman P, Brady HR. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int. 1997;51(5):1463–8.
Wuthrich RP. Intercellular adhesion molecules and vascular cell adhesion molecule-1 and the kidney. J Am Soc Nephrol. 1992;3(6):1201–11.
Henseleit U, Steinbrink K, Sunderkotter C, Goebeler M, Roth J, Sorg C. Expression of murine VCAM-1 in vitro and in different models of inflammation in vivo: correlation with immigration of monocytes. Exp Dermatol. 1995;4(5):249–56.
Seron D, Cameron JS, Haskard DO. Expression of VCAM-1 in the normal and diseased kidney. Nephrol Dial Transplant. 1991;6(12):917–22.
Wuthrich RP. Vascular cell adhesion molecule-1 (VCAM-1) expression in murine lupus nephritis. Kidney Int. 1992;42(4):903–14.
McLigeyo SO. Pathogenesis of lupus nephritis: a review. East Afr Med J. 1998;75(11):628–31.
Berden JH. Lupus nephritis. Kidney Int. 1997;52(2):538–58.
Walport MJ, Davies KA, Botto M. C1q and systemic lupus erythematosus. Immunobiology. 1998;199(2):265–85.
ter Borg EJ, Horst G, Hummel EJ, Limburg PC, Kallenberg CG. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum. 1990;33(5):634–43.
Bao L, Quigg RJ. Complement in lupus nephritis: the good, the bad, and the unknown. Semin Nephrol. 2007;27(1):69–80.
Wuthrich RP, Jevnikar AM, Takei F, Glimcher LH, Kelley VE. Intercellular adhesion molecule-1 (ICAM-1) expression is upregulated in autoimmune murine lupus nephritis. Am J Pathol. 1990;136(2):441–50.
Pallis M, Robson DK, Haskard DO, Powell RJ. Distribution of cell adhesion molecules in skeletal muscle from patients with systemic lupus erythematosus. Ann Rheum Dis. 1993;52(9):667–71.
Belmont HM, Buyon J, Giorno R, Abramson S. Up-regulation of endothelial cell adhesion molecules characterizes disease activity in systemic lupus erythematosus. The Shwartzman phenomenon revisited. Arthritis Rheum. 1994;37(3):376–83.
Ilic T, Mitic I, Durdevic-Mirkovic T, Vuckovic B, Milic B, Popovic M. Correlation between sera levels of sICAM-1 and sVCAM-1 and severity of kidney lesions in patients with lupus nephritis. Med Pregl. 2007;60(Suppl 2):128–32.
Sabry A, Sheashaa H, El-Husseini A, El-Dahshan K, Abdel-Rahim M, Elbasyouni SR. Intercellular adhesion molecules in systemic lupus erythematosus patients with lupus nephritis. Clin Rheumatol. 2007;26(11):1819–23.
Molad Y, Miroshnik E, Sulkes J, Pitlik S, Weinberger A, Monselise Y. Urinary soluble VCAM-1 in systemic lupus erythematosus: a clinical marker for monitoring disease activity and damage. Clin Exp Rheumatol. 2002;20(3):403–6.
Tan PL, Borman GB, Wigley RD. Testing clinical criteria for systemic lupus erythematosus in other connective tissue disorders. Rheumatol Int. 1981;1(3):147–9.
Berggård I, Bearn AG. Isolation and properties of a low molecular weight β2-globulin occurring in human biological fluids. J Biol Chem. 1968;243:4095–103.
Bazari H. Approach to the patient with renal disease. In: Goldman L, Ausiello D, editors. Cecil medicine. 23rd ed. Philadelphia: Saunders/Elsevier; 2007:chap 115.
Evenson MA. Report: criteria for analytical instrumentation in a clinical analysis laboratory. Anal Chem. 1979;51:1411A–3A.
Ledue TB, Johnson AM, Cohen LA, Ritchie RF. Evaluation of proficiency survey results for serum immunoglobulins following the introduction of a new international reference material for human serum proteins. Clin Chem. 1998;44:878–9.
Ledue TB, Weiner DL, Sipe JD, Poulin SE, Collins MF, Rifai N. Analytical evaluation of particle-enhanced immunonephelometric assays for C-reactive protein, serum amyloid A and mannose-binding protein in human serum. Ann Clin Biochem. 1998;35:745–53.
Tesar V, Masek Z, Rychlik I, Merta M, Bartunkova J, Stejskalova A, Zabka J, Janatkova I, Fucikova T, Dostal C, Becvar R. Cytokines and adhesion molecules in renal vasculitis and lupus nephritis. Nephrol Dial Transplant. 1998;13(7):1662–7.
Wu T, Xie C, Wang HW, Zhou XJ, Schwartz N, Calixto S, Mackay M, Aranow C, Putterman C, Mohan C. Elevated urinary VCAM-1, P-selectin, soluble TNF receptor-1, and CXC chemokine ligand 16 in multiple murine lupus strains and human lupus nephritis. J Immunol. 2007;179(10):7166–75.
Spronk PE, Bootsma H, Huitema MG, Limburg PC, Kallenberg CG. Levels of soluble VCAM-1, soluble ICAM-1, and soluble E-selectin during disease exacerbations in patients with systemic lupus erythematosus (SLE); a long term prospective study. Clin Exp Immunol. 1994;97(3):439–44.
Tesar V, Masek Z, Rychlik I, Merta M, Bartunkova J, Stejskalova A, Zabka J, Fucikova T, Dostal C, Becvar R. Cytokines and adhesion molecules in renal vasculitis and lupus nephritis. Cas Lek Cesk. 1997;136(16):501–6.
Arrizabalaga Clemente P. Adhesion molecules and glomerulonephritis. Towards novel therapeutic strategies. Med Clin (Barc). 2002;119(4):145–8.
Wang XC, Feng J, Huang F, Fan YS, Wang YY, Cao LY, Wen CP. Effects of Shikonin isolated from Zicao on lupus nephritis in NZB/W F1 mice. Biol Pharm Bull. 2009;32(9):1565–70.
Brey RL, Amato AA, Kagan-Hallet K, Rhine CB, Stallworth CL. Anti-intercellular adhesion molecule-1 (ICAM-1) antibody treatment prevents central and peripheral nervous system disease in autoimmune-prone mice. Lupus. 1997;6(8):645–51.
Kootstra CJ, Van Der Giezen DM, Van Krieken JH, De Heer E, Bruijn JA. Effective treatment of experimental lupus nephritis by combined administration of anti-CD11a and anti-CD54 antibodies. Clin Exp Immunol. 1997;108(2):324–32.
Kuroiwa T, Lee EG. Cellular interactions in the pathogenesis of lupus nephritis: the role of T cells and macrophages in the amplification of the inflammatory process in the kidney. Lupus. 1998;7(9):597–603.
Brady HR. Leukocyte adhesion molecules: potential targets for therapeutic intervention in kidney diseases. Curr Opin Nephrol Hypertens. 1993;2(2):171–82.
Christopher-Stine L, Siedner M, Lin J, Haas M, Parekh H, Petri M, Fine DM. Renal biopsy in lupus patients with low levels of proteinuria. J Rheumatol. 2007;34:332–5.
Acknowledgments
No funding was received from outside sources.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Abd-Elkareem, M.I., Al Tamimy, H.M., Khamis, O.A. et al. Increased urinary levels of the leukocyte adhesion molecules ICAM-1 and VCAM-1 in human lupus nephritis with advanced renal histological changes: preliminary findings. Clin Exp Nephrol 14, 548–557 (2010). https://doi.org/10.1007/s10157-010-0322-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-010-0322-z