Abstract
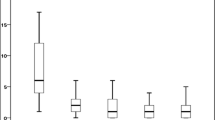
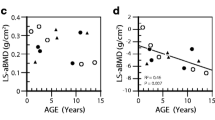
Osteogenesis imperfecta (OI) is a common genetic disorder that manifests with intrauterine or pre- or postnatal fractures, blue sclera, and deafness. Various treatments for the management of OI have been tried, of which bisphosphonates (BPs) seem to have the maximum benefit in reducing fracture rate and improving bone density. Zolendronic acid is a newer BP tried for several bone diseases, mainly in adults. The objective of our analysis was to study the response to zolendronic acid in children with type III OI. The case records of subjects with type III OI receiving zolendronic acid in the past 3 years between February 2006 and March 2009 were analyzed. Relevant details were recorded on a predesigned chart. Subjective improvement, reduction in number of fractures, and the DEXA scan Z-score were used to judge improvement. Five OI type III cases were followed up in the Genetic clinic. Presentation was from neonatal period to 7 years of age; M:F ratio was 3:2. Average duration of therapy given was 20.4 months. Improvement was noted in all patients, in the form of reduction in frequency of fractures (P = 0.002) and increase in bone density on DEXA scan (P = 0.01). Side effects noted were flu-like symptoms and myalgia. No clinical problems due to hypocalcemia were noted in any of the patients. Thus, zolendronic acid is seen as a safe and effective BP in type III OI children. The exact dose for optimal benefit is yet to be determined. The long-term effects of newer BPs need further long-term trials.


Similar content being viewed by others
References
Romero R, Pilu GL, Jeanty P (1988) Prenatal diagnosis of congenital anomalies. Appleton & Lange, Norwalk
Rauch F, Glorieux FH (2004) Osteogenesis imperfecta. Lancet 363:1377–1385
Plotkin H, Primorac D, Rowe D (2003) Osteogenesis imperfecta. In: Glorieux FH, Pettifor J, Jueppner H (eds) Pediatric bone. Academic Press, San Diego, pp 443–471
Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R (1998) Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med 339:947–952
Di Meglio LA, Peacock M (2006) Two-year clinical trial of oral alendronate versus intravenous pamidronate in children with osteogenesis imperfecta. J Bone Miner Res 21:132–140
Reid IR, Brown JP, Burckhardt P, Horowitz Z, Richardson P, Trechsel U et al (2002) Intravenous zolendronic acid in postmenopausal women with low bone mineral density. N Engl J Med 346:653–661
Reid IR, Miller P, Lyles K, Fraser W, Brown JP, Saidi Y, Mesenbrink P, Su G, Pak J, Zelenakas K, Luchi M, Richardson P, Hosking D (2005) Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. N Engl J Med 353:898–908
Lipton A, Cook R, Saad F, Major P, Garnero P, Terpos E, Brown JE, Coleman RE (2008) Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer 113:193–201
Rauch F, Glorieux FH (2005) Bisphosphonate treatment in osteogenesis imperfecta: which drug, for whom, for how long? Ann Med 37:295–302
Sykes B, Ogilvie D, Wordsworth P, Wallis G, Mathew C, Beightn P, Nicolls A, Pope FM, Thompson E, Tsipouras P, Schwartz R, Jensson O, Arnason A, Børresen AL, Heiberg A, Frey D, Steinmann B (1990) Consistent linkage of dominantly inherited osteogenesis imperfecta to the type I collagen loci: COL1A1 and COL1A2. Am J Hum Genet 46:293–307
Glorieux FH, Travers R, Chabot G, Lanoue G (1994) Bone histomorphometric analysis in osteogenesis imperfecta. J Bone Miner Res 9:S226 (abstract)
Brown JJ, Zacharin MR (2009) Safety and efficacy of intravenous zolendronic acid in paediatric osteoporosis. J Pediatr Endocrinol Metab 22:55–63
Li EC, Davis LE (2003) Zoledronic acid: a new parenteral bisphosphonate. Clin Ther 25:2669–2708
Plotkin H, Nunez M, Alvarez Filgueira ML, Zanchetta JR (1996) Lumbar spine bone density in Argentine children. Calcif Tissue Int 58:144–149
Davie MW, Haddaway MJ (1994) Bone mineral content and density in healthy subjects and in osteogenesis imperfecta. Arch Dis Child 70:331–334
Reinus WR, McAlister WH, Schranck F, Chines A, Whyte MP (1998) Differing lumbar vertebral mineralization rates in ambulatory pediatric patients with osteogenesis imperfecta. Calcif Tissue Int 62:17–20
Vetter U, Pontz B, Zauner E, Brenner RE, Spranger J (1992) Osteogenesis imperfecta: a clinical study of the first ten years of life. Calcif Tissue Int 50:36–41
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Panigrahi, I., Das, R.R., Sharda, S. et al. Response to zolendronic acid in children with type III osteogenesis imperfecta. J Bone Miner Metab 28, 451–455 (2010). https://doi.org/10.1007/s00774-009-0149-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-009-0149-4