Abstract
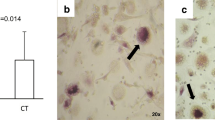
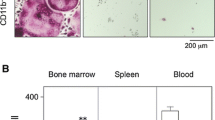
Osteolytic disorders cause serious problems for quality of life with aging. Osteolysis is performed by osteoclasts of the hematopoietic lineage that share some characteristics with monocytes and macrophages. As osteoclast precursors (pOCs) are present in peripheral blood, their characterization in osteolytic diseases may help us to understand risk factors. Although essential factors for osteoclastogenesis have been reported, the effective induction from pOCs in human peripheral blood mononuclear cells (PBMCs) to mature osteoclasts in culture requires further improvement. The aim of this study was development of an efficient culture system for human osteoclastogenesis and providing a simple system for the enrichment of pOCs from PBMCs. We employed coculturing of human PBMCs with a mouse stromal cell line. Significant numbers of tartrate-resistant acid phosphatase-positive (TRAP+) multinucleated osteoclasts (MNCs), which could resorb dentine slices, were efficiently induced in this culture condition. pOCs were enriched in an anti-CD16 antibody column-passed anti-CD14 antibody-bound cell population isolated by magnetic cell sorting. We compared the percentage of the CD14high CD16dull cell population, which mainly contained pOCs in PBMCs, from age-matched patients with rheumatoid arthritis (RA) and osteoporosis (OP), but it was comparable. However, the mean number of TRAP+ MNCs generated in cultures from PBMCs of RA was higher. In contrast, the frequency of pOCs in PBMCs from OP was relatively higher. These results suggest the characteristics of pOCs from RA and OP may be different, because single pOCs from OP gave rise to lower numbers of osteoclasts than those from RA.


Similar content being viewed by others
References
Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289:1508–1514
Mundy GR (1999) Bone remodeling and its disorders, 2nd edn. Martin Dunitz, London
Walsh NC, Crotti TN, Goldring SR, Gravallese EM (2005) Rheumatic diseases: the effects of inflammation on bone. Immunol Rev 208:228–251
Clowes JA, Riggs BL, Khosla S (2005) The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev 208:207–227
Goldring SR, Granallese EM (2000) Pathogenesis of bone erosions in rheumatoid arthritis. Curr Opin Rheumatol 12:195–199
American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines (2002) Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum 46:328–346
De Martinis M, Di Benedetto MC, Mengoli LP, Ginaldi L (2006) Senile osteoporosis: is it an immune-mediated disease? Inflamm Res 55:399–404
Suda T, Udagawa N, Takahashi N (1996) Cells of bone: osteoclast generation. In: Bilezikian JP, Raisz LG, Roden GA (eds) Principles of bone biology. Academic Press, New York, pp 87–102
Hayashi SI, Yamane T, Miyamoto A, Hemmi H, Tagaya H, Tanio Y, Kanda H, Yamazaki H, Kunisada T (1998) Commitment and differentiation of stem cells to the osteoclast lineage. Biochem Cell Biol 76:911–922
Fujikawa Y, Quinn JM, Sabokbar A, McGee JO, Athanasou NA (1996) The human osteoclast precursor circulates in the monocyte fraction. Endocrinology 137:4058–4060
Yoshida H, Hayashi SI, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature (Lond) 345:442–444
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602
Theill LE, Boyle WJ, Penninger JM (2002) RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol 20:795–823
Lagasse E, Weissman IL (1997) Enforced expression of Bcl-2 in monocytes rescues macrophages and partially reverses osteopetrosis in op/op mice. Cell 89:1021–1031
Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, Kanno T, Murakami A, Tsuda E, Morinaga T, Higashio K (1998) Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139:1329–1337
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Hirayama T, Danks L, Sabokbar A, Athanasou NA (2002) Osteoclast formation and activity in the pathogenesis of osteoporosis in rheumatoid arthritis. Rheumatology 41:1232–1239
Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM (1999) Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature (Lond) 402:304–309
Carlsten H (2005) Immune responses and bone loss: the estrogen connection. Immunol Rev 208:194–206
Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, Choi Y (2006) Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol 24:33–63
Tanaka S, Nakamura K, Takahasi N, Suda T (2005) Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol Rev 208:30–49
Tsurukai T, Udagawa N, Matsuzaki K, Takahashi N, Suda T (2000) Roles of macrophage-colony stimulating factor and osteoclast differentiation factor in osteoclastogenesis. J Bone Miner Metab 18:177–184
Holmes SG, Still K, Buttle DJ, Bishop NJ, Grabowski PS (2004) Chemically modified tetracyclines act through multiple mechanisms directly on osteoclast precursors. Bone 35:471–478
Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T (1989) The bone marrow-derived stromal cell line MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology 125:1805–1813
Yamada T, Yamazaki H, Yamane T, Yoshino M, Okuyama H, Tsuneto M, Kurino T, Hayashi SI, Sakano S (2003) Regulation of osteoclast development by Notch signaling directed to osteoclast precursors and through stromal cells. Blood 101:2227–2234
Kollet O, Dar A, Lapidot T (2007) The multiple roles of osteoclasts in host defense: bone remodeling and hematopoietic stem cell mobilization. Annu Rev Immunol 25:51–69
Takayanagi H (2005) Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med 83:170–179
Massey HM, Flanagan AM (1999) Human osteoclasts derived from CD14-positive monocytes. Br J Haematol 106:167–170
Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC (1990) CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431–1433
Unkeless J (1989) Function and heterogeneity of human Fc receptors for immunoglobulin G. J Clin Invest 83:355–361
Nicholson GC, Malakellis M, Collier FM, Cameron PU, Holloway WR, Gough TJ, Gregorio-king C, Kirkland MA, Myers DE (2000) Induction of osteoclasts from CD14-positive human peripheral blood mononuclear cells by receptor activator of nuclear factor κB ligand (RANKL). Clin Sci 99:133–140
Komano Y, Nanki T, Hayashida K, Taniguchi K, Miyasaka N (2006) Identification of a human peripheral blood monocyte subset that differentiates into osteoclasts. Arthritis Res Ther 8:R152
Shevde N, Anklesaria P, Greenberger JS, Bleiberg I, Glowacki J (1994) Stromal cell-mediated stimulation of osteoclastogenesis. Proc Soc Exp Biol Med 205:306–315
Tamura T, Takahashi N, Akatsu T, Sasaki T, Udagawa N, Tanaka S, Suda T (1993) New resorption assay with mouse osteoclast-like multinucleated cells formed in vitro. J Bone Miner Res 8:953–960
Hayashi SI, Miyamoto A, Yamane T, Kataoka H, Ogawa M, Sugawara S, Nishikawa S, Nishikawa S, Sudo T, Yamazaki H, Kunisada T (1997) Osteoclast precursors in bone marrow and peritoneal cavity. J Cell Physiol 170:241–247
Yang CR, Wang JH, Hsieh SL, Wang SM, Hsu TL, Lin WW (2004) Decoy receptor 3 (DcR3) induces osteoclast formation from monocyte/macrophage lineage precursor cells. Cell Death Differ 11:S97–S107
Hofmann G, Bernabei PA, Crociani O, Cherubini A, Guasti L, Pillozzi S, Lastraioli E, Polvani S, Bartolozzi B, Solazzo V, Gragnani L, Defilippi P, Rosati B, Wanke E, Olivotto M, Arcangeli A (2001) HERG K+ channels activation during β1 integrin-mediated adhesion to fibronectin induces an up-regulation of αvβ3 integrin in the preosteoclastic leukemia cell line FLG 29.1. J Biol Chem 276:4923–4931
Schoeler D, Grützkau A, Henz BM, Küchler J, Krüger-Krasagakis S (2003) Interleukin-6 enhances whereas tumor necrosis factor α and interferons inhibit integrin expression and adhesion of human mast cells to extracellular matrix proteins. J Invest Dermatol 120:795–801
Swanson C, Lorentzon M, Conaway HH, Lemer UH (2006) Glucocorticoid regulation of osteoclast differentiation and expression of receptor activator of nuclear factor-kappaB (NF-κB) ligand, osteoprotegerin, and receptor activator of NF-κB in mouse calvarial bones. Endocrinology 147:3613–3622
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature (Lond) 423:337–342
Quinn JM, Neale S, Fujikawa Y, McGee JO, Athanasou NA (1998) Human osteoclast formation from blood monocytes, peritoneal macrophages, and bone marrow cells. Calcif Tissue Int 62:527–531
Ross FP, Teitelbaum SL (2005) αvβ3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol Rev 208:88–105
Maekawa TL, Takahashi TA, Fujihara M, Urushibara N, Kadowaki-Kikuchi E, Nishikawa M, Ikebuchi K, Asano S, Ozawa K, Sekiguchi S (1997) A novel gene (drad-1) expressed in hematopoiesis-supporting stromal cell lines, ST2, PA6 and A54 preadipocytes: use of mRNA differential display. Stem Cells 15:334–339
Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T (2004) Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature (Lond) 428:758–763
Ziegler-Heitbrock L (2007) The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 81:584–592
Acknowledgments
We thank Drs. N. Takakura (Osaka University) and M. Yoshino (Tottori University) for helpful suggestions, M. Takahashi (Otsuka Pharmaceutical Co. Ltd) for M-CSF, and N. Udagawa (Matsumoto Dental University) for dentine slices. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and from the Molecular Medical Science Institute, Otsuka Pharmaceutical Co., Ltd, Tokushima, Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nose, M., Yamazaki, H., Hagino, H. et al. Comparison of osteoclast precursors in peripheral blood mononuclear cells from rheumatoid arthritis and osteoporosis patients. J Bone Miner Metab 27, 57–65 (2009). https://doi.org/10.1007/s00774-008-0011-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-008-0011-0