Abstract
Background
Randomised, controlled trials (RCT) and systematic reviews of RCT with meta-analysis are considered to be of highest methodological quality and therefore are given the highest level of evidence (Ia/b). Although, “low-quality” RCT may be downgraded to level of evidence IIb, the methodological quality of each individual RCT is not respected in detail in this classification of the level of evidence.
Materials and methods
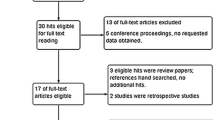
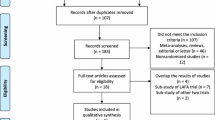
Within a systematic Cochrane Review of RCT on short-term benefits of laparoscopic or conventional colorectal resections, the methodological quality of all included RCT was evaluated. All RCT were assessed by the Evans and Pollock questionnaire (E and P increasing quality from 0–100) and the Jadad score (increasing quality from 0–5).
Results
Publications from 28 RCT printed from 1996 to 2005 were included in the analysis. Methodological quality of RCT was only moderate [E & P 55 (32–84); Jadad 2 (1–5)]. There was a significant correlation between the E & P and the Jadad score (r = 0.788; p < 0.001). Methodological quality of RCT slightly increased with increasing number of patients included (r = 0.494; p = 0.009) and year of publication (r = 0.427; p = 0.03). Meta-analysis of all RCT yielded clinically relevant differences for overall and local morbidity when compared to meta-analysis of “high-quality” (E & P > 70) RCT only.
Conclusion
The methodological quality of reports of RCT comparing laparoscopic and open colorectal resection varies considerably. In a systematic review, methodological quality of RCT should be assessed because meta-analysis of “high-quality” RCT may yield different results than meta-analysis of all RCT.




Similar content being viewed by others
References
Sackett DL (1986) Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest 89(2):S2–S3
Cook DJ, Guyatt GH, Laupacis A, Sackett DL (1992) Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest 102(4):S305–S311
Oxford Centre for Evidence-Based Medicine (2005) Levels of evidence and grades of recommendation. Centre for Evidence-Based Medicine; http://www.cebm.net/levels_of_evidence.asp#levels.
Schwenk W, Haase O, Neudecker J, Muller JM (2005) Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev (3):CD003145
Basse L, Jakobsen DH, Bardram L, Billesbolle P, Lund C, Mogensen T et al (2005) Functional recovery after open versus laparoscopic colonic resection: a randomized, blinded study. Ann Surg 241(3):416–423
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith A et al. (2005) Short-term endpoints of conventional versus laparoscopic assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
The Colon Cancer Open or Laparoscopic Study Group (COLOR) (2005) Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 6:477–484
Evans M, Pollock AV (1985) A score system for evaluating random control clinical trials of prophylaxis of abdominal surgical wound infection. Br J Surg 72:256–260
Jadad A, Moore A (1996) Assessing the quality of reports of randomized clinical trials: Is blindness necessary? Control Clin Trials 17:1–12
Alderson P, Green S, Higgins JPT (2004) Cochrane Reviewer’s Handbook 4.2.2. Wiley, Chichester, UK, (updated March 2004)
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Spilker B (1991) Guide to clinical trials, 1st ed. Raven Press
Moher D, Jadad AR, Tugwell P (1996) Assessing the quality of randomized controlled trials. Current issues and future directions. Int J Technol Assess Health Care 12(2):195–208
Moher D, Jadad AR, Nichol G, Penman M, Tugwell P, Walsh S (1995) Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials 16(1):62–73
Emerson JD, Burdick E, Hoaglin DC, Mosteller F, Chalmers TC (1990) An empirical study of the possible relation of treatment differences to quality scores in controlled randomized clinical trials. Control Clin Trials 11(5):339–352
Schulz KF, Chalmers I, Hayes RJ, Altman DG (1995) Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273(5):408–412
Plint AC, Moher D, Morrison A, Schulz K, Altman DG, Hill C et al (2006) Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust 185(5):263–267
Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M et al. (1998) Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 352(9128):609–613
Conflict of interest and funding
The authors state that there are no commercial or other associations that might pose a conflict of interest in connection with submitted material. There was no external funding of any kind supporting the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was presented during the Surgical Forum of 122nd Congress of the German Surgical Society in Berlin in May 2006.
Rights and permissions
About this article
Cite this article
Schwenk, W., Haase, O., Günther, N. et al. Methodological quality of randomised controlled trials comparing short-term results of laparoscopic and conventional colorectal resection. Int J Colorectal Dis 22, 1369–1376 (2007). https://doi.org/10.1007/s00384-007-0318-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-007-0318-7