Abstract
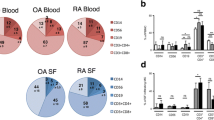
The objective of the present study was to assess the prevalences of naive, memory, memory/effector, regulatory and activated T-cells in peripheral blood (PB) and synovial fluid (SF) of patients with spondyloarthritis (SpA), rheumatoid arthritis (RA), polymyalgia rheumatica/giant cell arteritis (PMR/GCA) and healthy controls (HC). Twenty-two patients with SpA, 15 patients with RA, 38 patients with PMR/GCA and 17 HC were prospectively enrolled. The expression of differentiation and activation markers (CD3, CD4, CD8, CD25, CD28, CD45RA, CD45RO) characterizing T-cell subsets were analyzed by flow cytometry. The frequency of CD3+CD4+CD28− memory/effector T-cells was increased in PB of patients with SpA (median 1.1%, range 0.1–69.6), RA (2.5%, 0–42.9) and PMR/GCA (2.7%, 0–49.5) when compared with HC (0.7%, 0–38.0) and tended to be higher in SF of SpA patients (4.5%, 0.2–7.2, P = 0.084). CD28+CD45RA+CD4+ (9.6%, 4.1–10.3) and CD28+CD45RA+CD8+ naive T-cells (15.0%, 12.9–26.2) were reduced and CD28+CD45RO+CD4+ (93.5%, 51.0–99.0), CD28+CD45RO+CD8+ memory (81.2%, 38.9–83.5), CD8+CD25+ activated T-cells (10.9%, 2.7–13.8) and CD4+CD25hi TREGs (10.2%, 7.0–13.3) were increased in SF compared to PB (P < 0.05 each). These findings demonstrate altered T-cell subsets in patients with immune-mediated disease, particularly at sites of inflammation.


Similar content being viewed by others
References
Kang YM, Zhang X, Wagner UG, Yang H, Beckenbaugh RD, Kurtin PJ, Goronzy JJ, Weyand CM (2002) CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med 195:1325–1336. doi:10.1084/jem.20011565
Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM (2001) T cell activation in rheumatoid synovium is B cell dependent. J Immunol 167:4710–4718
Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O’Fallon WM, Goronzy JJ, Weyand CM (2001) Lymphoid neogenesis in rheumatoid synovitis. J Immunol 167:1072–1080
Byrne JA, Butler JL, Cooper MD (1988) Differential activation requirements for virgin and memory T cells. J Immunol 141:3249–3257
Kohem CL, Brezinschek RI, Wisbey H, Tortorella C, Lipsky PE, Oppenheimer-Marks N (1996) Enrichment of differentiated CD45RBdim, CD27− memory T cells in the peripheral blood, synovial fluid, and synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum 39:844–854. doi:10.1002/art.1780390518
Morita Y, Yamamura M, Kawashima M, Harada S, Tsuji K, Shibuya K, Maruyama K, Makino H (1998) Flow cytometric single-cell analysis of cytokine production by CD4+ T cells in synovial tissue and peripheral blood from patients with rheumatoid arthritis. Arthritis Rheum 4:1669–1676. doi:10.1002/1529-0131(199809)41:9<1669::AID-ART19>3.0.CO;2-G
Brunner-Weinzierl MC, Hoff H, Burmester GR (2004) Multiple functions for CD28 and cytotoxic T lymphocyte antigen-4 during different phases of T cell responses: implications for arthritis and autoimmune diseases. Arthritis Res Ther 6:45–54. doi:10.1186/ar1158
Kremer JM (2005) Selective costimulation modulators: a novel approach for the treatment of rheumatoid arthritis. J Clin Rheumatol 11:55–62. doi:10.1097/01.rhu.0000166626.68898.17
Schmidt D, Goronzy JJ, Weyand CM (1996) CD4+ CD7− CD28− T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest 97:2027–2037. doi:10.1172/JCI118638
Goronzy JJ, Weyand CM (2001) Thymic function and peripheral T-cell homeostasis in rheumatoid arthritis. Trends Immunol 22:251–255. doi:10.1016/S1471-4906(00)01841-X
Tarazona R, DelaRosa O, Alonso C, Ostos B, Espejo J, Pena J, Solana R (2000) Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev 121:77–88. doi:10.1016/S0047-6374(00)00199-8
Weyand CM, Goronzy JJ (1999) T-cell responses in rheumatoid arthritis: systemic abnormalities-local disease. Curr Opin Rheumatol 11:210–217. doi:10.1097/00002281-199905000-00010
Prelog M (2006) Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev 5:136–139. doi:10.1016/j.autrev.2005.09.008
Raffeiner B, Dejaco C, Duftner C, Kullich W, Goldberger C, Vega SC, Keller M, Grubeck-Loebenstein B, Schirmer M (2005) Between adaptive and innate immunity: TLR4-mediated perforin production by CD28null T-helper cells in ankylosing spondylitis. Arthritis Res Ther 7:R1412–R1420. doi:10.1186/ar1840
Abedin S, Michel JJ, Lemster B, Vallejo AN (2005) Diversity of NKR expression in aging T cells and in T cells of the aged: the new frontier into the exploration of protective immunity in the elderly. Exp Gerontol 40:537–548. doi:10.1016/j.exger.2005.04.012
Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA (2001) CD4+CD25high regulatory cells in human peripheral blood. J Immunol 167:1245–1253
Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M (2005) A peripheral circulating compartment of natural naive CD4+ Tregs. J Clin Invest 115:1953–1962. doi:10.1172/JCI23963
Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M (2006) Imbalance of regulatory T cells in human autoimmune diseases. Immunology 117:289–300. doi:10.1111/j.1365-2567.2005.02317.x
Su CC, Shau WY, Wang CR, Chuang CY, Chen CY (1997) CD69 to CD3 ratio of peripheral blood mononuclear cells as a marker to monitor systemic lupus erythematosus disease activity. Lupus 6:449–454. doi:10.1177/096120339700600507
Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, Cats A, Dijkmans B, Olivieri I, Pasero G et al (1991) The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 34:1218–1227. doi:10.1002/art.1780341003
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324. doi:10.1002/art.1780310302
Bird HA, Esselinckx W, Dixon AS, Mowat AG, Wood PH (1979) An evaluation of criteria for polymyalgia rheumatica. Ann Rheum Dis 38:434–439. doi:10.1136/ard.38.5.434
Duftner C, Dejaco C, Kullich W, Klauser A, Goldberger C, Falkenbach A, Schirmer M (2006) Preferential type 1 chemokine receptors and cytokine production of CD28− T-cells in ankylosing spondylitis. Ann Rheum Dis 65:647–653. doi:10.1136/ard.2005.042085
Duftner C, Goldberger C, Falkenbach A, Würzner R, Falkensammer B, Pfeiffer KP, Maerker-Hermann E, Schirmer M (2003) Prevalence, clinical relevance and characterization of circulating cytotoxic CD4+CD28− T cell in ankylosing spondylitis. Arthritis Res Ther 5:292–300. doi:10.1186/ar793
Namekawa T, Snyder MR, Yen JH, Goehring BE, Leibson PJ, Weyand CM, Goronzy JJ (2000) Killer cell activating receptors function as costimulatory molecules on CD4+CD28null T cells clonally expanded in rheumatoid arthritis. J Immunol 165:1138–1145
Lamprecht P, Moosig F, Csernok E, Seitzer U, Schnabel A, Mueller A, Gross WL (2001) CD28 negative T cells are enriched in granulomatous lesions of the respiratory tract in Wegener’s granulomatosis. Thorax 56:751–757. doi:10.1136/thorax.56.10.751
Markovic-Plese S, Cortese I, Wandinger KP, McFarland HF, Martin R (2001) CD4+CD28− costimulation-independent T cells in multiple sclerosis. J Clin Invest 108:1185–1194
Liuzzo G, Kopecky SL, Frye RL, O’Fallon WM, Maseri A, Goronzy JJ, Weyand CM (1999) Perturbation of the T-cell repertoire in patients with unstable angina. Circulation 100:2135–2139
Duftner C, Seiler R, Klein-Weigel P, Gobel H, Goldberger C, Ihling C, Fraedrich G, Schirmer M (2005) High prevalence of circulating CD4+CD28− T-cells in patients with small abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 25:1347–1352. doi:10.1161/01.ATV.0000167520.41436.c0
Nakajima T, Schulte S, Warrington KJ, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM (2002) T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation 105:570–575. doi:10.1161/hc0502.103348
Martens PB, Goronzy JJ, Schaid D, Weyand CM (1997) Expansion of unusual CD4+T cells in severe rheumatoid arthritis. Arthritis Rheum 40:1106–1114. doi:10.1002/art.1780400615
Goronzy JJ, Matteson EL, Fulbright JW, Warrington KJ, Chang-Miller A, Hunder GG, Mason TG, Nelson AM, Valente RM, Crowson CS, Erlich HA, Reynolds RL, Swee RG, O’Fallon WM, Weyand CM (2004) Prognostic markers of radiographic progression in early rheumatoid arthritis. Arthritis Rheum 50:43–54. doi:10.1002/art.11445
Gerli R, Schillaci G, Giordano A, Bocci EB, Bistoni O, Vaudo G, Marchesi S, Pirro M, Ragni F, Shoenfeld Y, Mannarino E (2004) CD4+CD28− T lymphocytes contribute to early atherosclerotic damage in rheumatoid arthritis patients. Circulation 109:2744–2748. doi:10.1161/01.CIR.0000131450.66017.B3
Warrington KJ, Kent PD, Frye RL, Lymp JF, Kopecky SL, Goronzy JJ, Weyand CM (2005) Rheumatoid arthritis is an independent risk factor for multi-vessel coronary artery disease: a case control study. Arthritis Res Ther 7:R984–R991. doi:10.1186/ar1775
Gupta S, Bi R, Su K, Yel L, Chiplunkar S, Gollapudi S (2004) Characterization of naive, memory and effector CD8+ T cells: effect of age. Exp Gerontol 39:545–550. doi:10.1016/j.exger.2003.08.013
Davila E, Kang YM, Park YW, Sawai H, He X, Pryshchep S, Goronzy JJ, Weyand CM (2005) Cell-based immunotherapy with suppressor CD8+ T cells in rheumatoid arthritis. J Immunol 174:7292–7301
Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM (2001) Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol 75:12182–12187. doi:10.1128/JVI.75.24.12182-12187.2001
Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, Parson W, Böck G, Schönitzer D, Trannoy E, Grubeck-Loebenstein B (2002) Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol 168:5893–5899
Ciubotariu R, Vasilescu R, Ho E, Cinti P, Cancedda C, Poli L, Late M, Liu Z, Berloco P, Cortesini R, Suciu-Foca Cortesini N (2001) Detection of T suppressor cells in patients with organ allografts. Hum Immunol 62:15–20. doi:10.1016/S0198-8859(00)00226-3
Schonland SO, Lopez C, Widmann T, Zimmer J, Bryl E, Goronzy JJ, Weyand CM (2003) Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc Natl Acad Sci USA 100:13471–13476. doi:10.1073/pnas.2233561100
Koetz K, Bryl E, Spickschen K, O’Fallon WM, Goronzy JJ, Weyand CM (2000) T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci USA 97:9203–9208. doi:10.1073/pnas.97.16.9203
Amyes E, McMichael AJ, Callan MF (2005) Human CD4+ T cells are predominantly distributed among six phenotypically and functionally distinct subsets. J Immunol 175:5765–5773
Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V (2004) CD25bright CD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther 6:R335–R346. doi:10.1186/ar1192
Liu MF, Wang CR, Fung LL, Wu CR (2004) Decreased CD4+CD25+ T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immunol 59:198–202. doi:10.1111/j.0300-9475.2004.01370.x
van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS (2004) CD4+CD25+ regulatory T cells in rheumatoid arthritis. Arthritis Rheum 50:2775–2785. doi:10.1002/art.20499
Cao D, Malmstrom V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C (2003) Isolation and functional characterization of regulatory CD25bright CD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol 33:215–223. doi:10.1002/immu.200390024
Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C (2004) Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med 200:277–285. doi:10.1084/jem.20040165
Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O (2005) CD4+CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol 140:360–367. doi:10.1111/j.1365-2249.2005.02754.x
Suarez A, Lopez P, Gomez J, Gutierrez C (2006) Enrichment of CD4+CD25high T cell population in SLE patients treated with glucocorticoids. Ann Rheum Dis 65:1512–1517. doi:10.1136/ard.2005.049924
Dejaco C, Duftner C, Schirmer M (2006) Are regulatory T-cells linked with aging? Exp Gerontol 41:339–345. doi:10.1016/j.exger.2006.01.008
Acknowledgments
This work was supported by the Innsbruck Medical University, the “Verein zur Förderung der wissenschaftlichen Ausbildung und Tätigkeit von Südtirolern an der Universität Innsbruck” (to C. Dejaco) and the Tyrolean Research Funds (to C. Duftner).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
296_2009_949_MOESM1_ESM.ppt
Exemplary fluorescence analysis of PB mononuclear cells stained with CD4 and CD25 mAbs for the analysis of CD4+CD25hi TREGs (according to reference 16). (A). Gates are set in the dot blot for CD4− (R1) and CD4+ (R2) T-cells. (B) Histograms are used to set the upper limit of CD25− T-cells according to the isotype control (filledblacksquare, *) and the upper limit of the CD25int T-cells according to the CD4−CD25+ cells (filledblackrectangle, +). Thus CD4+CD25int T-cells (including the activated CD4+ T-cells) and the CD4+CD25hi T-cells (representing the TREGs) can be differentiated. The gray area under the curve shows the distribution of CD25-expression on CD4+ T-cells (out of area R2), including the CD4+CD25hi TREGs. (C) Density blots show the different compartments after appropriate gating for CD4−CD25−and CD4−CD25int cells (out of the R1 area), and CD4+CD25−, CD4+CD25int and CD4+CD25hi cells (out of the R2 area). No cells can be detected in the CD4−CD25hi gate
Rights and permissions
About this article
Cite this article
Dejaco, C., Duftner, C., Klauser, A. et al. Altered T-cell subtypes in spondyloarthritis, rheumatoid arthritis and polymyalgia rheumatica. Rheumatol Int 30, 297–303 (2010). https://doi.org/10.1007/s00296-009-0949-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-009-0949-9