Abstract
Purpose
The incidence of osteoporosis-related fractures will increase substantially over the coming decades as the population ages globally. This has important economic and public health implications, contributing substantially to morbidity and excess mortality in this population.
Methods
When prescribing for older patients the effectiveness profile of drugs needs to be balanced against their tolerability in individual patients.
Results
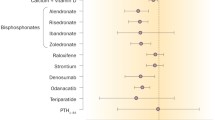
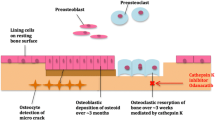
Currently we have good anti-fracture data to support the use of many available anti-resorptive and anabolic drugs including bisphosphonates, strontium ranelate and recombinant human parathyroid hormone. We also have evidence to demonstrate the importance of calcium and vitamin D repletion in these patients. However, in recent years our understanding of normal bone physiology and the mechanisms underlying the development of osteoporosis has significantly advanced and this has led to the development of new therapies. Novel agents, particularly denosumab, but also inhibitors of cathepsin K and anabolic agents that act on Wnt signalling, will increase the therapeutic options for clinicians in the coming years.
Conclusion
This review discusses the evidence supporting the use of currently available treatment options for osteoporosis and potential future advances in drug therapy. Particular consideration should be given when prescribing for certain older patients who have issues with compliance or tolerance and also in those with co-morbidities or levels of frailty that may restrict the choice of therapy. Understanding the evidence for the benefit and possible harm of osteoporosis treatments is critical to appropriate management of this patient population.


Similar content being viewed by others
References
Sambrook P, Cooper C (2006) Osteoporosis. Lancet 367:2010–2018
Cummings SR, Melton LJ (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet 359:1761
Heaney RP (2003) Remodelling and skeletal fragility. Osteoporos Int 14 [Suppl 5]:S12–S15
Bruyere O, Varela AR, Ademi S et al (2009) Loss of hip bone mineral density over time in association with spine and hip fracture incidence in osteoporotic postmenopausal women. Eur J Epidemiol 24(11):707–712
Seibel MJ, Naganathan V, Barton I et al (2004) Relationship between pretreatment bone resorption and vertebral fracture incidence in postmenopausal osteoporotic women treated with risedronate. J Bone Miner Res 19:323
Bauer DC, Black DM, Garnero P et al (2004) Change in bone turnover and hip, non-spine and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res 19:1250
Bauer DC, Garnero P, Hochberg MC et al (2006) Pretreatment levels of bone turnover and the antifracture efficacy of alendronate: the fracture intervention trial. J Bone Miner Res 21:292
Cummings SR, Nevitt MC, Browner WS et al (1995) Risk factors for hip fracture in white women. N Engl J Med 332:767
Kanis J et al (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385
Kanis JA, McCloskey EV, Johansson H et al (2008) Case finding for the management of osteoporosis with FRAX—assessment and intervention thresholds for the UK. National Osteoporosis Guideline Group. Osteroporos Int 19(10):1395–1408
Borgström F, Ström O, Coelho J et al (2010) The cost-effectiveness of risedronate in the UK for the management of osteoporosis using the FRAX. Osteoporos Int 21(3):495–505
Borgström F, Ström O, Coelho J et al (2010) The cost-effectiveness of strontium ranelate in the UK for the management of osteoporosis. Osteoporos Int 21(2):339–349
Hippisley-Cox J, Coupland C (2009) Predicating risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores. BMJ 339:b4229
Holick MF (2007) Optimal vitamin D status for the prevention and treatment of osteoporosis. Drugs Aging 24(12):1017–1029
Bischoff-Ferrari HA, Dietrich T, Orav E et al (2004) Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med 116:634
Jackson RD, LaCroix AZ, Gass M et al (2006) Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 354:669
Dawson-Hughes B, Harris SS, Krall EA et al (1997) Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 337:670
Prince RL, Devine A, Dhaliwal SS et al (2006) Effects of calcium supplementation on clinical fracture and bone structure. Results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Intern Med 166:869–875
Dawson-Hughes B, Bischoff-Ferrari HA, Mayer J (2007) Therapy of osteoporosis with calcium and vitamin D. J Bone Miner Res 22 [Suppl 2]:V59–V63
Bolland MJ, Avenell A, Baron J et al (2010) Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ 341:c3691
Dunford JE (2010) Molecular targets of the nitrogen containing bisphosphonates: the molecular pharmacology of prenyl synthase inhibition. Curr Pharm Des 16(27):2961–2969
Black DM, Cummings SR, Karpf DB et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348(9041):1535–1541
Cummings SR, Black DM, Thompson DE et al (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures. Results from the fracture intervention trial. JAMA 280:2077
Harris ST, Watts NB, Genant HK et al (1999) Effects of risedronate treatment on vertebral and non-vertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 282:1344
McClung MR, Geusens P, Miller PD et al (2001) Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 344:333
Delmas PD, Recker RR, Chesnut CH III et al (2004) Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int 15(10):792–798
Harris ST, Reginster JY, Harley C et al (2009) Risk of fracture in women treated with monthly oral ibandronate or weekly bisphosphonates: the eValuation of IBandronate Efficacy (VIBE) database fracture study. Bone 44(5):758–765
Black DM, Delmas PD, Eastell R et al (2007) Once-yearly zolendronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809
Lyles KW, Colon-Emeric CS, Magaziner JS et al (2007) Zolendronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357:1799
Eriksen EF, Lyles KW, Colón-Emeric CS et al (2009) Antifracture efficacy and reduction of mortality in relation to timing of the first dose of zoledronic acid after hip fracture. Bone Miner Res 24(7):1308–1313
Wysowski DK (2009) Reports of esophageal cancer with oral bisphosphonate use. N Engl J Med 360(1):89–90
Cardwell CR, Abnet CC, Cantwell MM et al (2010) Exposure to oral bisphosphonates and risk of esophageal cancer. JAMA 304(6):657–663
Green J, Czanner G, Reeves G et al (2010) Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ 341:c4444
Black DM, Kelly MP, Genant HK et al (2010) Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med 363(19):1761–1771
Abrahamsen B, Eiken P, Eastell R (2010) Cumulative alendronate dose and the long-term absolute risk of subtrochanteric and diaphyseal femur fractures: a register-based national cohort analysis. J Clin Endocrinol Metab 95(12):5258–5265
CHMP assessment report on bisphosphonate and osteonecrosis of the jaw (2009). www.emea.europa.eu
Rossouw JE, Anderson GL, Prentice RL et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 288(3):321–333
(1996) Effects of hormone therapy on bone mineral density: results from the postmenopausal estrogen/progestin interventions (PEPI) trial. The Writing Group for the PEPI. JAMA 276(17):1389–1396
Ettinger B, Black DM, Mitlak BH et al (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282(7):637–645
Delmas PD, Genant HK, Crans GG et al (2003) Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone 33(4):522–532
Silverman SL, Christiansen C, Genant HK et al (2008) Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res 23(12):1923–1934
Cummings SR, Ensrud K, Delmas PD et al (2010) PEARL study investigators. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med 362(8):686–696
Komi J, Lankinen KS, DeGregorio M et al (2006) Effects of ospemifene and raloxifene on biochemical markers of bone turnover in postmenopausal women. J Bone Miner Metab 24(4):314–318
Bolognese M, Krege JH, Utian WH et al (2009) Effects of arzoxifene on bone mineral density and endometrium in postmenopausal women with normal or low bone mass. J Clin Endocrinol Metab 94(7):2284–2289
Black DM, Greenspan SL, Ensrud KE et al (2003) The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207
Neer RM, Arnaud CD, Zanchetta JR et al (2001) Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434
Vahle JL, Sato M, Long GG et al (2002) Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol 30:312
Black DM, Bilezikian JP, Ensrud KE et al (2005) One year of alendronate after one year of parathyroid hormone (PTH 1-84) for osteoporosis. N Engl J Med 353:555
Meunier PJ, Roux C, Seeman E et al (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis (SOTI). N Engl J Med 350(5):459–468
Seeman E, De Vernejoul MC, Adami S et al (2005) Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab 90(5):2816–2822
Reginster JY, Bruyère O, Sawicki A et al (2009) Long-term treatment of postmenopausal osteoporosis with strontium ranelate: results at 8 years. Bone 45(6):1059–1064
Seeman E, Vellas B, Benhamou C et al (2006) Strontium ranelate reduces the risk of vertebral and nonvertebral fractures in women eighty years of age and older. J Bone Miner Res 21(7):1113–1120
Bruyere O, Roux C, Detilleux J et al (2007) Relationship between bone mineral density changes and fracture risk reduction in patients treated with strontium ranelate. J Clin Endocrinol Metab 92(8):3076–3081
Breart G, Cooper C, Meyer O et al (2010) Osteoporosis and venous thromboembolism: a retrospective cohort study in the UK General Practice Research Database. Osteoporos Int 21(7):1181–1187
Lewiecki EM (2008) Denosumab: an investigational drug for the management of postmenopausal osteoporosis. Biologics 2(4):645–653
McClung MR, Lewiecki EM, Cohen SB et al (2006) AMG 162 Bone Loss Study Group. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354(8):821–831
Lewiecki EM, Miller PD, McClung MR et al (2007) Two year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res 22(12):1832–1841
Miller PD, Bolognese MA, Lewiecki EM et al (2008) Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomised blinded phase 2 clinical trial. Bone 43(2):222–229
Brown JP, Prince RL, Deal C et al (2009) Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 24(1):153–161
Cummings SR, San Martin J, McClung MR et al (2009) FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361(8):756–765
Aghaloo T, Felsenfeld A, Tetradis S (2010) Osteonecrosis of the jaw in a patient on denosumab. J Oral Maxillofac Surg 68(5):959–963
Taylor KH, Middlefell LS, Mizen KD (2010) Osteonecrosis of the jaws induced by anti-RANK ligand therapy. Br J Oral Maxillofac Surg 48(3):221–223
Kendler DL, McClung MR, Freemantle N, on behalf of the DAPS Investigators et al (2010) Adherence, preference, and satisfaction of postmenopausal women taking denosumab or alendronate. Osteoporos Int doi:10.1007/s00198-010-1378-z
Gauthier JY, Chauret N, Cromlish W et al (2008) The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg Med Chem Lett 18(3):923–928
Stoch SA, Zajic S, Stone J et al (2009) Effect of the cathepsin K inhibitor odanacatib on bone resorption biomarkers in healthy postmenopausal women: two double-blind, randomized, placebo-controlled phase I studies. Clin Pharmacol Ther 86(2):175–182
Bone HG, McClung MR, Roux C (2010) Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J Bone Miner Res 25(5):937–947
Henriksen DB, Alexandersen P, Hartmann B et al (2007) Disassociation of bone resorption and formation by GLP-2. A 14 day study in healthy postmenopausal women. Bone 40(3):723
Henriksen DB, Alexandersen P, Hartmann B et al (2009) Four month treatment with GLP-2 significantly increases hip BMD: a randomized, placebo-controlled, dose-ranging study in postmenopausal women with low BMD. Bone 45(5):833–842
Hoeppner LH, Secreto FJ, Westendorf JJ (2009) Wnt signaling as a therapeutic target for bone diseases. Expert Opin Ther Targets 13(4):485–496
Li X, Ominsky MS, Warmington KS et al (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24(4):578–588
Padhi D, Jang G, Stouch B, Fang L et al (2010) Single-dose, placebo-controlled, randomised study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26(1):19–26
Anastasilakis AD, Polyzos SA, Avramidis A (2010) The effect of teriparatide on serum Dickkopf-1 levels in postmenopausal women with established osteoporosis. Clin Endocrinol (Oxf) 72(6):752–757
MacDonald BT, Joiner DM, Oyserman SM et al (2007) Bone mass is inversely proportional to Dkk1 levels in mice. Bone 41(3):331–339
Betts AM, Clark TH, Yang J et al (2010) The application of target information and preclinical pharmacokinetic/ pharmacodynamic modeling in predicting clinical doses of a Dickkopf-1 antibody for osteoporosis. J Pharmacol Exp Ther 333(1):2–13
Nugent C, Roche K, Wilson S et al (2009) The effect of intramuscular vitamin D (cholecalciferol) on serum 25OH vitamin D levels in older female acute hospital admissions. Ir J Med Sci 179(1):57–61
Harwood RH, Sahota O, Gaynor K et al (2004) Nottingham Neck of Femur (NONOF) Study. A randomised, controlled comparison of different calcium and vitamin D supplementation regimens in elderly women after hip fracture: The Nottingham Neck of Femur (NONOF) Study. Age Ageing 33(1):45–51
Seeman E, Devogelaer JP, Lorenc R et al (2008) Strontium ranelate reduces the risk of vertebral fractures in patients with osteopenia. J Bone Miner Res 23:433–438
Quandt SA, Thompson DE, Schneider DL et al (2005) Effect of alendronate on vertebral fracture risk in women with bone mineral density T scores of −1.6 to −2.5 at the femoral neck. Mayo Clin Proc 80:343–349
Miller PD, Roux C, Boonen S et al (2005) Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. J Bone Miner Res 20:2105–2115
Jamal SA, Bauer DC, Ensrud KE et al (2007) Alendronate treatment in women with normal to severely impaired renal function: an analysis of the fracture intervention trial. J Bone Miner Res 22:503–508
Ishani A, Blackwell T, Jamal SA et al (2008) MORE investigators. The effect of raloxifene treatment in postmenopausal women with CKD. J Am Soc Nephrol 19:1430–1438
Bergner R, Hendrich D, Hoffmann M et al (2008) Treatment of reduced bone density with ibandronate in dialysis patients. J Nephrol 21(4):510–516
Wetmore JB, Benet LZ, Kleinstuck D et al (2005) Effects of short-term alendronate on bone mineral density in haemodialysis patients. Nephrology (Carlton) 10(4):393–399
Lane NE, Yao W (2009) Developments in the scientific understanding of osteoporosis. Arthritis Res Ther 11:228
Kanis JA, McCloskey EV, Johansson H, Oden A, Ström O, Borgström F (2010) Development and use of FRAX® in osteoporosis. Osteoporos Int 21 [Suppl 2]:407–413
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brewer, L., Williams, D. & Moore, A. Current and future treatment options in osteoporosis. Eur J Clin Pharmacol 67, 321–331 (2011). https://doi.org/10.1007/s00228-011-0999-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-0999-2