Article Text
Abstract
Aim To investigate the therapeutic value of azathioprine as monotherapy or combined with other immunosuppressive drugs for uveitis in patients with juvenile idiopathic arthritis (JIA).
Methods A retrospective multicentre study including 41 children with JIA (28 (68.2%) female) with unilateral or bilateral (n=28) chronic anterior uveitis. Azathioprine was used to treat uveitis that was active in patients receiving topical or systemic corticosteroids, methotrexate or other immunosuppressive drugs. The primary end point was assessment of uveitis inactivity. Secondary end points comprised dose sparing of topical steroids and systemic corticosteroids, and immunosuppression.
Results At 1 year, uveitis inactivity was achieved in 13/17 (76.5%) patients by using azathioprine as systemic monotherapy and in 5/9 (56.6%) as combination therapy. During the entire azathioprine treatment period (mean 26 months), inactivity was obtained in 16/26 patients (61.5%) with monotherapy and in 10/15 (66.7%) when combined with other immunosuppressives (p=1.0). With azathioprine, dosages of systemic immunosuppression and steroids could be reduced by ≥50% (n=12) or topical steroids reduced to ≤2 drops/eye/day in six patients. In three patients (7.3%), azathioprine was discontinued because of nausea and stomach pain.
Conclusions Azathioprine may be reconsidered in the stepladder approach for the treatment of JIA-associated uveitis. The addition of azathioprine may also be beneficial for patients not responding properly to methotrexate.
- Azathioprine
- immunosuppression
- juvenile idiopathic arthritis
- uveitis
Statistics from Altmetric.com
Introduction
Juvenile idiopathic arthritis (JIA) is the most common chronic inflammatory rheumatic disease in childhood and begins before 16 years of age. JIA is characterised by a chronic inflammation of the joints, which may lead to joint destruction.1–3 The most frequent form of eye involvement in JIA is uveitis, with an estimated incidence of between 10% and 18%.4 5 Uveitis is characterised by chronic inflammation of the iris and ciliary body. The course of the disease varies greatly. A severe and vision-threatening course develops in approximately one-third of patients, and the complication rate rises during the chronic course of the disease.5–7
The aim of treatment is to completely abolish active inflammation in the eye. Treatment is usually started with topical corticosteroids, occasionally combined with systemic corticosteroids. However, in ∼30% of patients the uveitis remains active, so that immunosuppressive drugs are required. Methotrexate is the first-choice immunosuppressive; ciclosporin A, azathioprine or the tumor necrosis factor α (TNFα) inhibitors represent other options.
Azathioprine is a well-known purine synthesis inhibitor that is widely used in organ transplantation and several autoimmune diseases, for example rheumatoid arthritis. A favourable response has also previously been noted in the treatment of arthritis in patients with JIA.8 9
Azathioprine has only occasionally been utilised to treat uveitis in patients with JIA. Indeed, only small case series have been reported in the literature, describing experience with azathioprine in only a few children.9 10 Here we summarise our observations using azathioprine in treating 41 patients with JIA-associated uveitis.
Patients and methods
All patients in this study fulfilled the classification of JIA in agreement with the International League of Associations for Rheumatology (ILAR) criteria.3 The 41 children were treated with azathioprine for severe chronic anterior uveitis. The cohort included consecutive patients who were started on azathioprine treatment for uveitis between 1995 and 2007. Charts were reviewed retrospectively. The design of the work conforms to the standards currently applied in Germany. No institutional review board approval is required for chart review studies.
Rheumatological examinations and diagnostic procedures included a review of systems, laboratory tests (eg, antinuclear antibodies (ANAs), rheumatoid factor and human leucocyte antigen (HLA)-B27), urine analysis, and chest x-ray, if necessary. An infectious aetiology was excluded if serological tests for Epstein–Barr virus (EBV), cytomegalovirus (CMV), herpes simplex virus (HSV), herpes zoster virus (HZV), Treponema, Toxoplasma and Toxocara, and the tuberculosis skin test were negative.
Patients with other uveitis entities, such as intermediate, Fuchs heterochromic cyclitis and infectious uveitis syndromes, were excluded from the study. A standardised ophthalmic database was applied for the analysis. The ophthalmic assessment included best-corrected visual acuities (BCVAs), slit-lamp examination, tonometry (Goldmann) and funduscopy.
Uveitis was defined and anatomically classified according to the Standardisation of Uveitis Nomenclature (SUN) Working Group recommendations.11 The presence of ≥1+ cells in the anterior chamber was defined as active uveitis. We documented the duration of uveitis and whether disease was unilateral or bilateral. Dates of first and final azathioprine treatments were noted, as were azathioprine dosage, adverse effects, any topical and anti-inflammatory medication before and during azathioprine treatment, and initial manifestation of uveitis in the fellow eye after beginning azathioprine treatment. Additionally, the uveitis-related complications in the eye were analysed, as central band-keratopathy, new synechiae formation, cataract, vitreous opacity, phthisis and glaucoma. Data were collected from all patients at 6–12 month intervals.
Azathioprine monotherapy was instituted in patients with active uveitis, if they were being treated with topical (≥3 drops/day) and possibly also systemic corticosteroids (≥10 mg, or ≥0.15 mg/kg body weight), or in order to spare systemic corticosteroids. Moreover, azathioprine was considered as an additional immunosuppressive if uveitis remained active during treatment with any other immunosuppressive drug.
The dosages of systemic corticosteroids and immunosuppressive drugs were altered or medication discontinued according to the clinical assessment of arthritis and uveitis activity and to the medication-related side effects.
After 1 year of azathioprine treatment and at all visits during the entire treatment period, the response to treatment, as defined by inactivity of uveitis, and non-response, as defined by activity of uveitis, were analysed. Inactivity of anterior uveitis was defined as <1+ cells in the anterior chamber.11 Response to treatment with azathioprine was additionally judged according to a modified grading system described by Saurenmann et al12 Briefly, a good response was defined as a decrease of ≥50% in both corticosteroid use and immunosuppressives (if not on combined immunosuppressive drug and systemic corticosteroid at institution of azathiopine, then only reduction of corticosteroids of ≥50%); moderate response, reduction of ≥50% in either corticosteroid or immunosuppressive drug; and poor response, reduction of <50% in both corticosteroid and immunosuppressive drug. In this study, dose sparing was assessed independently for systemic and topical steroids.
All analyses were performed using MedCalc software (MedCalc Version 9.6.4.0; Mariakerke, Belgium). The t test, χ2 test and Fisher exact test for categorical data were applied for statistical analysis when appropriate. A p value <0.05 was considered as a significant difference.
Results
A total of 41 children were included in this study. All children had JIA and associated active chronic anterior uveitis. Demographic characteristics including JIA subgroups and uveitis are listed in table 1. Notably, the majority of patients suffered from early-onset arthritis and showed a severe course of uveitis, with complications already present at the initial visit in nearly half of the patients, for example central band-keratopathy (n=5), cataract (n=20), vitreous opacity (n=5) and glaucoma (n=12). All of the patients had previously received topical corticosteroids, and 29.3% had received immunosuppressive drugs. The follow-up after instituting azathioprine was 26 months (mean, range 3–107). Azathioprine was started at a mean dosage of 2.4 mg/kg body weight (range 1.4–3.2). Then, azathioprine was continued, and the mean dosage at the end of the study period was 2.1 mg/kg body weight daily (range 1.0–2.8) (p=0.047).
Patient chracteristics before treatment with azathioprine of children with uveitis associated with juvenile idiopathic arthritis (JIA)
Effect of azathioprine monotherapy on uveitis
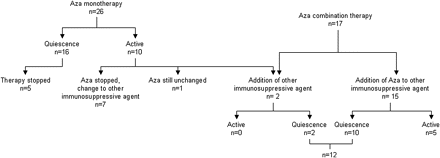
Of the 26 patients who initially received azathioprine monotherapy (figure 1) for uveitis, inactivity of uveitis was achieved in 16 (61.5%), while inflammation recurred or persisted in the other 10 (38.5%). Mean follow-up was 26 months (table 2). Response to treatment did not correlate well with JIA subgroup (p=1.0), age at onset of arthritis (p=0.76), ANA positivity (p=1.0), gender (p=0.12), age at onset of uveitis (p=0.41), bilateral uveitis (p=1.0) and presence of complications at initial presentation (p=1.0).
Azathioprine (Aza) for uveitis in 41 children with juvenile idiopathic arthritis.
Azathioprine monotherapy as initial immunosuppressive treatment (n=26)
A significant interpatient variation was observed in the dose-sparing effect of immunosuppressive drugs and topical steroids. New uveitis complications were noted in 3 of 26 patients in at least one eye during the treatment period (table 2).
Effect of azathioprine in combination with other disease-modifying antirheumatic drugs on uveitis
Of the 41 patients included, azathioprine was given in combination with other immunosuppressive drugs in 15 patients (table 3 and figure 1). While azathioprine was added to previous immunosuppression in 13 patients, other immunosuppressive drugs were then added to the regimen in another two patients with active uveitis receiving azathioprine. Whereas in seven patients, azathioprine was combined with methotrexate, azathioprine was combined with ciclosporin A in five patients and with other immunosuppressive drugs (Leflunomid, Adalimumab, Etanercept) in another three patients. In 10 of 15 patients (66.7%) treated with azathioprine in combination with other immunosuppressive drugs, inactivity of uveitis was achieved. It is note worthy that the response to azathioprine treatment analysed at 1 year and for the entire period (mean 24 months) did not differ from that of the group of patients treated for uveitis with azathioprine monotherapy (p=1.0)
Azathioprine when added to treatment of patients already on any immunosuppressive drugs (n=15)
The outcome of azathioprine in combination with other immunosuppressive drugs with regard to sparing the dose of the other systemic immunosuppressive agents or corticosteroids was good in six, moderate in four, and poor in five patients (table 3). In this respect, the difference between the azathioprine monotherapy and combination therapy failed to reach the level of significance (p=0.23). In this group, topical steroids could be tapered off in six patients, while the dosage was unchanged in another nine. The sparing effect of topical steroids was not better than in the monotherapy group (p=0.42).
The observations with azathioprine given in combination with methotrexate or ciclosporin A, which are commonly used to treat uveitis associated with JIA, are summarised in table 4 and 5. Although the numbers of patients in both of the groups are fairly small, the observations suggest that uveitis inactivity may be achieved with the combined use of methotrexate or ciclosporin A with azathioprine. However, the sparing effect of topical and systemic corticosteroids and immunosuppressive drugs was moderate to poor in the majority of cases.
Azathioprine when added to treatment of patients already on methotrexate (n=7)
Azathioprine when added to treatment of patients already on ciclosporin A (n=5)
New complications and side effects under azathioprine
Twenty patients (49%) had already developed uveitis complications before azathioprine treatment was instituted. However, the first or additional uveitis complications were noted in another seven patients while receiving azathioprine treatment.
During the treatment period with azathioprine monotherapy, 3 of the 26 patients (11.5%) developed new secondary complications. Four of the 15 patients receiving systemic combination treatment (26.7%) were reported to have developed new uveitis complications, and these numbers did not differ from the azathioprine monotherapy group (p=0.41).
Azathioprine was withdrawn in three patients because of nausea. Apart from this, no other serious side effects were documented during the study period.
Discussion
Only very few small case series have assessed the value of azathioprine to treat uveitis in patients with associated JIA.8 9 The observations from our multicentre study show that this agent may be helpful as systemic monotherapy or in combination with other immunosuppressive agents. Azathioprine may also be useful for long-term treatment and shows a dose-sparing effect for corticosteroids or immunosuppression.
The pathogenesis of uveitis in patients with JIA is not well defined. The few published histopathological papers demonstrated that the disease is marked by non-granulomatous inflammation in the iris and ciliary body.13–15 A recently published paper showed that the cellular infiltration mostly consisted of plasma cells, plasmocytic lymphocytes and monocytes, and few T cells. In light of these findings, the poor response to ciclosporin A that was described most recently is not surprising, suggesting that JIA uveitis may not be primarily a T cell-mediated disease.16 The focal aggregates mainly consisted of CD20+ B cells: primarily immunoglobulin G (IgG) B cells and only a few IgM B cells.17 However, as the infiltrate at nucleation may not reflect that during early active disease, the therapeutic implications of end-stage histology are limited.
Azathioprine is a purine nucleoside analogue. It interferes with adenine and guanine ribonucleotides by suppressing inosinic acid synthesis, which in turn interferes with DNA replication and RNA transcription.18 Immunologically, azathioprine decreases the numbers of peripheral T and B lymphocytes and reduces mixed lymphocyte reactivity, interleukin 2 synthesis and IgM production.19 20 In addition, the production of cytokines such as interferon γ and TNF in T lymphocytes is inhibited and apoptosis of these cells is initiated.21
Azathioprine is well absorbed orally and cleaves to 6-mercaptopurine, which is metabolised to thioinosinic and thioguanylic acid by the enzyme hypoxanthine phosphoribosyltransferase. These metabolites affect ribonucleotide synthesis.22 Therefore, azathioprine appears to be an appropriate candidate drug for uveitis in JIA. Azathioprine has also been introduced for the treatment of uveitis associated with JIA.8 9
Savolainen et al9 reported that in the long term azathioprine could effectively treat arthritis in 129 patients with JIA. In this study, a total of 19 patients had chronic iridocyclitis in some phase of the disease. Three children had episodes before treatment with azathioprine and 14 patients had active uveitis when included in study. In the latter cases activity persisted during treatment. Iridocyclitis occurred for the first time in two patients during low-dose therapy. In contrast to our study, no specific time points were analysed, the cell grade in the anterior chamber as a measure for activity was not specified as currently agreed, nor was the adjustment of the topical steroid treatment considered.11 It may be speculated that additional topical treatment was not adjusted properly to the inflammatory activity.
Hemady et al examined the role of immunosuppressive drugs in the management of progressive, corticosteroid-resistant uveitis associated with JIA.10 The mean duration of immunosuppressive treatment was 6.4 months. Only 4 of 26 patients were treated with azathioprine. In two patients inflammation was controlled. The other two patients—one receiving azathioprine in combination with methotrexate—did not respond to the treatment.
In contrast to the rare published data, our observations in a larger and homogenous group of patients with JIA suggest that azathioprine may be effective in the treatment of JIA-associated chronic anterior uveitis. During the mean follow-up of 26 months with azathioprine monotherapy, uveitis inactivity was maintained in 16 of 26 children (61.5%). The sparing effect of anti-inflammatory medication, however, differed markedly among the individuals. The effectiveness of other immunosuppressive drugs in the treatment of JIA-associated uveitis has also been investigated only in retrospective uncontrolled studies. In one study including 23 patients, remission was described in 417 of 661 months with methotrexate treatment.23 In another study, quiescence of uveitis was obtained with methotrexate in 32 of 35 patients.24 Inactivity of uveitis was noted with ciclosporin A in only 6 of 25 patients.16
The impact of azathioprine on the visual outcome could not be determined in this study, as the documented visual acuities were frequently unreliable due to children's non-compliance or because of opacities in the visual axis. Although this case series did not include a control group, the data reveal that new uveitis complications occurred in some patients during the treatment period. Indeed, new complications were detected in 3 of the 26 patients (11.5%) included, and in two of the patients the first uveitis manifestation developed in the formerly uninvolved eye although they were being treated with azathioprine.
Currently, the first-choice immunosuppressive drug is methotrexate, which is quite effective in controlling uveitis in patients with JIA.23 24 The management of children with JIA with uveitis who do not respond to methotrexate represents an important issue, as this is the most frequently used immunosuppressant in these patients. It is therefore noteworthy that our data show that azathioprine was not less effective when it was added to an immunosuppressive drug already being given. When azathioprine was added to methotrexate in the children with active uveitis in our case series, inactivity was achieved in 71.4%. However, our observations show that the sparing effect of the other systemic immunosuppressive drugs and of topical steroids can vary. For the entire patient series, systemic immunosuppressive drugs and steroids could be reduced only in a minority of patients. It is possible that the similar outcomes with azathioprine monotherapy and combination therapy are due to the differences in severity between the groups. However, uveitis activity, duration of uveitis and complication rates did not differ between the groups (data not shown).
Although we could speculate that higher azathioprine dosages would have given better results in this study, the azathioprine dosages did not differ significantly between the responders and the non-responders (monotherapy p=0.3 and combined therapy p=1.0). According to safety recommendations, the dosage for this patient group should be <3 mg/kg body weight. It has been shown previously that azathioprine treatment at this dosage is safe and well tolerated in children.25
Apart from the anti-inflammatory value of immunosuppressive agents, drug safety is of the outmost importance in children with JIA with uveitis, especially as long-term treatment is usually required. Thus, long-term safety data are needed, which is a good reason for preferentially using particular drugs, for example methotrexate, ciclosporin A and also azathioprine. Therefore, the use of azathioprine may be favoured in patients who do not respond to methotrexate, and may be an alternative to some recently introduced drugs, for example TNFα inhibitors, leflunomide or mycofenolate mofetil.
In summary, the present data suggest that azathioprine may be reconsidered in the stepladder approach for the treatment of children with JIA-associated uveitis. Although this study is somewhat limited by its retrospective design and short follow-up in some patients, the results suggest that the value of azathioprine monotherapy is higher than often believed. The addition of azathioprine may also be beneficial for patients not responding properly to other immunosuppressive drugs. Prospective randomised studies and long-term registries are necessary to define the most effective and safest treatment regimen.
References
Footnotes
Competing interests None.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.