Article Text
Abstract
Background The licensed dose of rituximab in rheumatoid arthritis (RA) is two doses of 1000 mg given 2 weeks apart. A lower dose has never been specifically studied in patients with an inadequate response to anti-tumour necrosis factor (TNF) agents.
Objective To compare the efficacy and safety of rituximab repeat treatment with two doses (1000 mg×1 and 1000 mg×2) following initial treatment with 1000 mg×2.
Methods We set up an open-label, prospective, multicentre, non-inferiority study comprising a non-controlled period (24 weeks) followed by a randomised controlled period (weeks 24–104) in patients with RA and an inadequate response to anti-TNF agents. All patients received one course of rituximab (1000 mg×2) with methotrexate. At week 24, patients achieving a EULAR response (moderate or good) were randomised to rituximab retreatment at 1000 mg×1 (Arm A) or 1000 mg×2 (Arm B). The primary objective measure was disease activity in 28 joints C-reactive protein (DAS28-CRP) area under the curve (AUC) over 104 weeks with a non-inferiority margin defined by 20% (444) of the mean DAS28-CRP AUC (mean±SD 2218±967) of the reference data.
Results The intent-to-treat and per-protocol (PP) populations comprised 143 (A/B: 70/73) and 100 (A/B: 51/49) patients, respectively. The adjusted mean difference in DAS28-CRP AUC (PP) was 51.4 (95% CI −131.2 to 234), demonstrating non-inferiority between arms A and B. The overall rituximab safety profile was similar with both retreatment regimens.
Conclusions Following a clinical response to a first course of rituximab in RA at the licensed dose of 1000 mg×2, retreatment with rituximab at 1000 mg×1 results in efficacy outcomes that are non-inferior to those achieved with retreatment at 1000 mg×2.
ClinicalTrials.gov registration number: NCT01126541.
- Rheumatoid Arthritis
- B cells
- Treatment
Statistics from Altmetric.com
Introduction
Rheumatoid arthritis (RA) has a complex pathogenesis that requires individually and continuously tailored treatment for effective management. Methotrexate (MTX) has become the cornerstone of RA treatment; however, not all patients respond and loss of response over time is a problem. In such cases, biological therapy is indicated and tumour necrosis factor (TNF) inhibitors are frequently used as first-line biological agents. Primary or secondary resistance to TNF inhibitors is, however, not uncommon, with maintenance on therapy no more than 50% at 5 years.1 Thus, following TNF inhibitor failure, other biological therapies such as rituximab (RTX), abatacept or tocilizumab are used. In developed countries, 20–30% of patients with RA are treated with biological agents for life (TNF inhibitors or others) at an annual cost of between €10 000 and €15 000 per patient.2 The treatment of RA therefore has a significant socioeconomic impact in these countries.
Treatment with RTX, a B-cell depleting anti-CD20 monoclonal antibody, produces significant and clinically meaningful improvements in symptoms and protection of joints by slowing the progression of structural damage in patients with active RA who have had an inadequate response (IR) or were intolerant to TNF inhibitors.3 ,4 According to the licence,5 RTX should be administered intravenously as two 1000 mg infusions (with intravenous glucocorticoid premedication), separated by 2 weeks, with concomitant MTX. The need for retreatment is evaluated 24 weeks after the previous course and retreatment given should residual disease activity remain. Worldwide, more than 200 000 patients with RA have received RTX to date.6 The RTX licence and dose are based on the results of the REFLEX randomised controlled trial (RCT).3 Other phase III RCTs, conducted in MTX-IR (SERENE)7 and MTX-naïve patients (IMAGE),8 evaluated a dose of 500 mg×2 and reported very similar clinical and functional outcomes compared with the RTX 1000 mg×2 dose. Effects on joint damage were also assessed in MTX-naïve patients in the IMAGE study. The licensed dose (1000 mg×2) cohort demonstrated superior joint protection compared with the lower dose for the first year of treatment8; however, in an exploratory secondary analysis, both doses of RTX significantly reduced joint damage progression between 6 months and 1 year.8
The risk of severe infection is a crucial factor when assessing the risk:benefit ratio for biological drugs to treat autoimmune diseases such as RA. In the clinical trials performed with RTX in RA to date, a numerically higher rate of serious infections was seen in patients receiving RTX 1000 mg×2 compared with those receiving placebo: 4.7 vs 3.2 events per 100 patient-years (PY) in the DANCER study9 and 5.2 vs 3.7 events per 100 PY in the REFLEX study.3 Across previous studies, the rate of serious infections was comparable between dose groups (1000 mg×2 and 500 mg×2), with the exception of the DANCER study, and also comparable to those reported for TNF inhibitor therapy.7–13
Thus, further data evaluating the risk:benefit ratio of a lower RTX dose regimen and its economic impact are warranted. A widely used treatment approach across other therapeutic fields is to use a ‘loading dose’ to produce rapid symptomatic improvement followed by maintenance retreatment at a lower dose. To test this approach for RTX in RA, we conducted a randomised trial (SMART) comparing the safety and efficacy of retreatment with RTX 1000 mg×1 or 1000 mg×2 following an induction treatment of 1000 mg×2 in TNF-IR patients. The study was designed to assess non-inferiority between the two retreatment regimens.
Methods
Study design
This was a prospective multicentre national study consisting of a 24-week non-controlled period followed by a randomised open-label controlled period from weeks 24 to 104. The primary objective was to assess the efficacy (disease activity in 28 joints C-reactive protein (DAS28-CRP) area under the curve (AUC)) of two dose regimens of RTX over 104 weeks in patients who had a EULAR response (good or moderate) 6 months after a first course of RTX (two infusions of 1000 mg) and who required retreatment. Secondary objectives included comparing the efficacy of the two dosing regimens with regard to DAS28; American College of Rheumatology (ACR) 20/50/70; European League Against Rheumatism (EULAR) response; number of required courses of RTX; time to second retreatment; and safety.
Setting and participants
SMART was conducted at 44 centres in France between September 2006 and November 2011. Adult outpatients (>18 years of age) with RA for ≥6 months (1987 ACR definition)14 and an inadequate response, intolerance or contraindication to etanercept, infliximab or adalimumab were enrolled. Patients were required to have discontinued etanercept for ≥4 weeks and infliximab or adalimumab for ≥8 weeks prior to randomisation and to be receiving stable doses of MTX (≥10 mg/week) for ≥4 weeks. Corticosteroids (≤10 mg/day prednisone or equivalent) and non-steroidal anti-inflammatory drugs were permitted if at stable doses for ≥4 and ≥2 weeks prior to screening, respectively.
The main exclusion criteria were: rheumatic autoimmune disease other than RA; concurrent treatment with any TNF inhibitor therapy or biological therapy; significant cardiac or pulmonary disease (investigator's decision); significant uncontrolled disease (investigator's decision); known active infection or history of serious recurrent or chronic infection; laboratory findings: hepatitis B surface antigen positive, hepatitis C virus positive, haemoglobin <8 g/dl, neutrophil count <1.5×103/µl, IgM <0.4 g/l and/or IgG <5 g/l.
Randomisation and interventions
At week 0 eligible patients received the first course of RTX (two 1000 mg infusions given on days 1 and 15). At week 24 the investigator was in charge of calculating whether the patient fulfilled the EULAR response criteria response (moderate or good). If no, the patients withdrew from the trial. If yes, the patients were randomly allocated to retreatment with one single infusion of RTX 1000 mg (Arm A) or RTX 1000 mg×2 given on days 1 and 15 (Arm B). Randomisation was conducted over the internet by a centralised balanced (1:1) adaptive randomisation process using a minimisation method and stratified according to centre and DAS at inclusion. All patients received premedication with intravenous methylprednisolone 100 mg 30 min before each infusion and continued MTX treatment at the dose current at the time of inclusion. After at least 24 weeks following the previous course, patients received retreatment based on evaluation of disease activity (DAS28 >3.2, segmented neutrophil count >1.5×103/µl and no contraindications); consultations were scheduled every 8 weeks for assessment of DAS28.
Outcomes and follow-up
Given the fact that the minimum time between retreatments was 6 months and that it is a 2-year strategy study, AUC seemed to us a more reliable end point than a single outcome at 2 years. Thus, the primary objective outcome measure was DAS28-CRP AUC over 104 weeks. Secondary objective outcome measures included: DAS28-CRP, EULAR response, ACR20/50/70 response, low disease activity (LDA), remission rates, tender joint count (TJC), swollen joint count (SJC), number of required courses of RTX, time to a second retreatment and quality of life. Parameters used to evaluate safety included adverse events (AEs), ECG, x-ray, laboratory assessments and human antichimeric antibodies (HACAs) against RTX (at baseline and before each RTX cycle).
Statistical analysis
Sample size calculation
One hundred and sixty-eight randomised patients (assuming 152 patients in the per-protocol (PP) population) were needed to demonstrate non-inferiority of Arm A versus Arm B for DAS28-CRP AUC over 104 weeks with 80% power, a significance level of α=2.5% (one-sided) and a non-inferiority margin defined by 20% (444) of the mean DAS28 AUC (mean±SD 2218±967) of the reference data.3 ,9 ,15
All randomised patients were included in the intent-to-treat (ITT) population. Patients were assigned to their initially assigned treatment arm irrespective of the treatment actually received. The PP population included all ITT patients without major protocol deviations. All patients who received at least one dose of study drug were included in the safety population.
Analysis of the primary endpoint was performed first on the PP and then on the ITT population. The AUC of DAS28-CRP values between day 1 and week 104 was calculated using the trapezoidal method. All AUC values were censored at week 104. Missing DAS28-CRP data were imputed by linear approximation (for missing values between two non-missing values) or by last observation carried forward (for premature withdrawal). The mean difference in AUC between the two treatment arms and its 95% CI were calculated using analysis of covariance, with AUC as the response variable and treatment arm and inclusion value of DAS28-CRP as covariates. If the 95% CI of the treatment difference (Arm A to Arm B) was within −∞ to 444, it was concluded that retreatment with RTX 1000 mg×1 (Arm A) was non-inferior to retreatment with RTX 1000 mg×2 (Arm B).
Based on previous reports which observed some discrepancies in categorising patients with RA by DAS28-ESR (erythrocyte sedimentation rate) and DAS28-CRP,16 an exploratory analysis assessing DAS28-ESR (AUC and change from baseline) was performed.
Analyses of secondary criteria were performed on the ITT and PP populations and no non-inferiority limits were predefined. Comparisons between the two arms were performed at the significance level of α=5% (two-sided) using the Student t test or non-parametric Wilcoxon test for quantitative data and the χ2 test or Fisher exact test for qualitative data. The Kaplan−Meier method was used to analyse time to retreatment. Logistic regressions were used to assess relationships between post-baseline hypo-IgG and occurrence of severe infections (first analysis) and between age and post-baseline hypo-IgG (second analysis).
Results
Patients
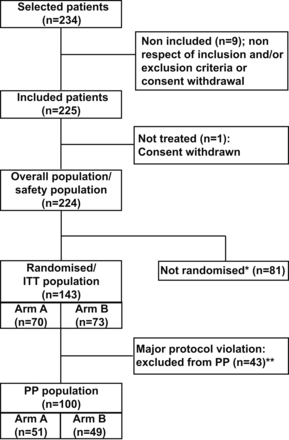
Patient disposition is shown in figure 1. Of 234 patients who met the inclusion criteria, 225 (96%) were included in the study and, of these, one patient did not receive RTX because of consent withdrawal. Thus, 224 patients were included in the overall and safety populations (including 81 patients who received one uncontrolled course of RTX and nine patients randomised but not retreated). Of the 224 patients, 143 were randomised (ITT population): 70 in Arm A and 73 in Arm B. The reasons for non-randomisation of the 81 other patients are indicated in figure 1. Forty-three patients (30%; 19 in Arm A and 24 in Arm B) had at least one major deviation from the protocol (mostly issues with compliance (no retreatment within 8 weeks following a DAS28-CRP >3.2), missing primary criterion due to premature withdrawal and change of MTX dose or route of administration). The PP population therefore comprised 100 patients (51 in Arm A and 49 in Arm B).
Study flow chart. Arm A: retreatment with one infusion of 1000 mg of RTX (day 1); Arm B: retreatment with two infusions of 1000 mg of RTX (day 1 and day 15). *Not randomised (n=81): premature withdrawal before week 24 (n=14), no EULAR response (n=64), affectation or situation aimed by the exclusion criteria defined by the protocol (n=6) (more than one reason is possible). **Major protocol violation (n=43): compliance (n=27), error of treatment arm compared with the arm given by the randomisation (n=1), intake of forbidden treatment (n=13), missing primary criterion (n=14) (more than one reason is possible). ITT, intent-to-treat; PP, per-protocol; RTX, rituximab.
Baseline patient characteristics were similar between the arms for both the ITT and PP populations (table 1). Non-randomised patients were less frequently rheumatoid factor positive than randomised patients (69% vs 90%).
Baseline characteristics (week 0: before rituximab initiation) in ITT population
Clinical efficacy
In the overall population, mean±DAS28-CRP at week 24 was 4.2±1.2; 71% of patients who achieved a EULAR response at this time point (22% good; 49% moderate) were randomised between the two retreatment groups. DAS28-CRP AUC (primary endpoint) between baseline and week 104 in the PP population (mean±SD) was similar between Arm A (2761±508) and Arm B (2666±490). The adjusted mean difference was 51.4 (95% CI –131.2 to 234) (see online supplementary table S1); as the upper limit of the 95% CI of the difference was below 444, non-inferiority was demonstrated (figure 2). Similar results were observed for the ITT population (adjusted mean difference –13.69 (95% CI –174.18 to 146.80) (see online supplementary table S1 and figure 2). DAS28-CRP weighted time AUC was also equivalent between the two groups both in the PP and in ITT analysis (figure 2).
Evolution of DAS28-CRP weighted time AUC and representation of the primary end point, adjusted difference DAS28-CRP AUC with 95% CI and the 20% margin, both in (A) PP and (B) ITT analysis. AUC, area under curve; DAS28-CRP, disease activity in 28 joints C-reactive protein; ITT, intent-to-treat; PP, per-protocol.
Results of the exploratory analysis of DAS28-ESR AUC between day 1 and week 104 were similar to those for DAS28-CRP AUC for both the PP and ITT populations (data not shown). Evolution of DAS28-CRP and DAS28-ESR (exploratory analysis) is shown in table 2. Mean DAS28-CRP was similar in both treatment arms at 24 weeks after each course of RTX and at first and second retreatments. The median time to first retreatment was 195 days (IQR 189–203). The median time to second retreatment was similar in both treatment arms: 263 days (IQR 227–294) in Arm A and 255 days (IQR 234–358) in Arm B. The median number of retreatments per year was 1.2 (range 0.6–1.7) with no difference between the two arms. In the ITT population, no statistically significant difference between the arms was observed for the other secondary endpoints: EULAR response, ACR response, TJC, SJC, CRP, ESR, LDA, remission rates and quality of life (data not shown).
Evolution of DAS28-CRP and DAS28-ESR in ITT population
Safety
The proportion of patients experiencing at least one treatment emergency AE was similar in the two randomised treatment groups and among non-randomised patients (table 3). A total of 1058 AEs were reported for 204 patients (91%) with no significant difference in the incidence between arms. Infections were reported in 125/224 (56%) patients. Among the subset of randomised patients, rates of infections were 70% in Arm A and 59% in Arm B; the corresponding incidence of serious infections was 12% and 3%, respectively (table 3). Overall, the most commonly occurring infections were bronchitis (18% of all patients), urinary tract infection (16%), gastroenteritis (9%), nasopharyngitis (7%), rhinitis (6%) and pharyngitis (5%). One case of hepatitis B reactivation was reported. Infection rates per 100 PY were 76.9, 89.6 and 71.2 in the total safety population, Arm A and Arm B, respectively; the corresponding rates of serious infections were 4.1, 7.1 and 1.5, respectively.
Summary of safety
Mean IgM and IgG levels decreased over time. IgG levels remained above the lower limit of normal (6.82 g/l) for most patients (≥95% in Arm A and ≥88% in Arm B) throughout the study. No difference was found in median changes in IgG levels between inclusion and week 104 across both arms (p=0.116; figure 3). No association was observed between post-baseline hypo-IgG and the occurrence of severe infection, or between age and the occurrence of post-baseline hypo-IgG.
Evolution of immunoglobulin G levels over time.
Three patients became HACA-positive during the study (two in Arm A and one in the non-randomised group); these patients reported several AEs, all of mild or moderate intensity with none occurring during RTX infusions. Overall, 33/224 patients (15%) reported infusion-related reactions (IRRs) during the first RTX infusion and 14 patients (6%) during the second infusion of the first course. One serious IRR occurred during the first infusion of the first course (angio-oedema leading to discontinuation of treatment). No serious IRRs occurred during retreatment and the rates of IRRs reduced after the first course with each subsequent infusion. One death was reported during the study. This was an accidental death occurring after treatment and was not related to the study drug.
Discussion
The results of this study clearly suggest that, in patients with RA who achieve a EULAR good/moderate response 6 months after a first course of RTX at the licensed dose of 1000 mg×2, retreatment with a single 1000 mg infusion is non-inferior to retreatment with a 1000 mg×2 dose regimen. With a follow-up of 2 years, both the mean improvement in DAS28 24 weeks after each RTX course (initial treatment and retreatment courses) and the median times to first and second retreatment were similar in both treatment arms.
Previous studies investigating the clinical efficacy of treatment with 1000 mg×2 and 500 mg×2 of RTX in MTX-naïve and DMARD-IR populations found no clinically relevant differences between the two regimens, although formal comparisons between the two doses were not performed.7 ,8 The main interest in using a reduced dose of RTX or, indeed, any biological agent is related to long-term safety. Although the tolerance of biological agents in RA is generally good, data from meta-analyses of RCTs or from registries, although controversial, suggest a slightly increased risk of severe infection with these agents. Risk factors for severe infections among ‘real-life’ patients enrolled in the French Autoimmunity in Rituximab (AIR) registry included chronic lung and/or cardiac disease, extra-articular involvement and low serum IgG levels before RTX.17 It is known that RTX retreatment may be associated with low IgG levels which, in turn, are potentially associated with an increased risk of serious infections.18 Few cases of opportunistic infections have been described in association with RTX treatment.19 ,20 The specific safety profile of RTX indicates an apparent absence of increased risk of reactivation of tuberculosis, a low risk of opportunistic infections, but possibly a small increased risk of the very rare but serious neurological infection progressive multifocal leukoencephalopathy (PML), the risk of which may be higher in patients with previous immunosuppression. As of May 2012, six cases of confirmed PML have been reported in patients with RA treated with RTX (Roche data on file); this complication is thus very rare (<1:25 000 treated patients with RA).
No increased risk of serious infections has been found in long-term follow-up of the RTX RCTs, even in patients who received more than five RTX courses.17 These data reflect the safety profile in patients who tolerate the drug and who have achieved good disease control (another risk factor of infections), including a reduced requirement for concomitant steroid medication.
RTX retreatment may be associated with the development of hypogammaglobulinaemia in some patients, mainly hypo-IgM but also hypo-IgG.17 In long-term follow-up of 3194 patients in the RTX RCTs with a mean follow-up of 9.5 years, 22.4% of patients developed hypo-IgM and 3.5% developed hypo-IgG for ≥4 months. The rate of serious infections in the 112 hypo-IgG patients was higher than in the 3082 patients without hypo-IgG (9.13 vs 3.72 per 100 PY), but was not different from the rate seen in the same 112 patients before the development of hypo-IgG (8.06 per 100 PY). However, it could not be excluded that the hypo-IgG was a risk factor of infections since these patients had normal but lower baseline IgG levels (8.4 g/l) compared with the other 3082 patients (13.2 g/l), and may have developed the hypo-IgG prior to the predefined sampling time and/or the recording of the first infection. Likewise, real-life data from the AIR registry showed that 4.6% of patients had hypo-IgG before RTX treatment while 8.2% of patients who had normal IgG levels at baseline developed hypo-IgG during RTX treatment. Interestingly, both categories of patient (baseline or acquired hypo-IgG) had an increased risk of serious infections (relative risk 4.9 (95% CI 1.6 to 15.2)).18 ,19 In the present study there was a numerically higher incidence of hypo-IgG in the two-infusion arm. Although there was no difference in the rate of infections or serious infections between the two arms at 2 years, it could not be excluded that, if the hypo-IgG was to persist beyond this time point, it could have consequences in terms of long-term risk of serious infections.
Using a reduced dose of RTX is also of interest because of the resultant economic benefits. Biological therapy is expensive and in some countries there is limited access to these drugs for economic reasons. A retreatment schedule of 1000 mg×1 also eliminates a day of hospitalisation. A single 1000 mg RTX infusion given every 9 months would cost less than €3000 in most countries, which is far less than for any other biological treatment.
This study has four main limitations. First, there is potential for physician and/or patient bias due to the open-label design. There is no obvious reason why patients or investigators would favour the single-infusion arm, although knowledge that the patient had received a lower dose of RTX may have increased the tendency to report relapse earlier in this arm. Second, the number of patients with major protocol deviations and therefore excluded from the PP primary analysis population was high, albeit with a similar number in each arm. As a consequence, this study could be considered as underpowered. However, analysis of the ITT population gave similar overall results. Third, the study primary end point is not validated. The rationale for choosing it has been driven by the fact that patients were to receive repeat treatments at different time points, thus triggering the need for a time-integrated outcome measure. Nevertheless, although not powered to detect a difference, the decrease in DAS28 after each repeat treatment and the time intervals between courses appeared to be similar between the two treatment groups. Finally, the study did not evaluate radiological outcomes; although clinical efficacy was not different between the arms, we cannot exclude the possibility that structural disease aspects could be differentially affected by the two dosing regimens.
In conclusion, in patients with RA who have achieved a EULAR response after a first course of RTX at the licensed dose of 1000 mg×2, retreatment using a reduced dose of 1000 mg×1 was not inferior to a 1000 mg×2 regimen at 2 years on disease activity. Retreatment at a reduced RTX dose may have a potential beneficial effect on the long-term risk of infections. Together with the known lack of association between RTX and opportunistic infections such as tuberculosis, this regimen might provide economic benefits and be an option for patients with RA in developing countries. Further studies are warranted in larger patient cohorts with inclusion of structural evaluation in order to confirm our findings.
Acknowledgments
The authors would like to thank the SMART investigators (all in France): Dr I Azais (Poitiers), Dr JC Balblanc (Belfort), Dr F Berenbaum (Paris), Dr P Bertin (Limoges), Dr M-C Boissier (Bobigny), Dr P Bourgeois (Paris), Dr A Cantagrel (Toulouse), Dr P Carli (Toulon), Dr P-Y Chouc (Marseille), Dr M Couret (Valence), Dr L Euller-Ziegler (Nice), Dr P Fardellone (Amiens), Dr P Fauquert (Berck/Mer), Dr R-M Flipo (Lille), Dr P Gaudin (Echirolles), Dr J-L Grauer (Aix en Provences), Dr A Heraud (Libourne), Dr P Hilliquin (Corbeil), Dr S Hoang (Vannes), Dr E Houvenagel (Lomme), Dr D Keita (Paris), Dr S Lassoued (Cahors), Dr L Le Dantec (Lievin), Dr J-M Le Parc (Boulogne), Dr L Lequen (Pau), Dr F Lioté, (Paris), Dr C Marcelli (Caen), Dr O Meyer (Paris), Dr J-L Pellegrin (Pessac), Dr A Perdriger (Rennes), Dr G Rajzbaum (Paris), Dr S Redeker (Abbeville), Dr J-M Ristori (Clermont-Ferrand), Dr A Saraux (Brest), Dr G Tanguy (La Roche sur Yon), Dr T Thomas (Saint-Priest-en-Jarez), Dr L Zabraniecki (Toulouse), Dr C Zarnitski (Montivilliers).
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online table
Footnotes
-
Handling editor Tore K Kvien
-
Contributors All authors contributed to the study design, analysis and interpretation of the data. XM and MD drafted the manuscript and all authors approved the final submission draft.
-
Funding The study was sponsored by Roche, France.
-
Competing interests SR and RJ are employees of Roche SAS. MD has acted as a consultant and received honoraria from Roche. JT, BC, JS, XM, XLeL and MD have served on advisory boards and received research grants from Roche.
-
Ethics approval The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference for Harmonisation (ICH) Harmonised Tripartite Guideline for Good Clinical Practices. The institutional review boards approved the study and all patients gave written informed consent before study entry.
-
Provenance and peer review Not commissioned; externally peer reviewed.