Article Text
Abstract
Objective Evaluate ustekinumab, an anti-interleukin (IL)-12 and IL-23 antibody, effects on radiographic progression in psoriatic arthritis (PsA).
Methods We conducted preplanned integrated analyses of combined radiographic data from PSUMMIT-1 and PSUMMIT-2 phase 3, randomised, controlled trials. Patients had active PsA despite prior conventional and/or biologic disease-modifying antirheumatic drugs (≥5/66 swollen, ≥5/68 tender joints, C-reactive protein ≥3.0 mg/L, documented plaque psoriasis). Patients (PSUMMIT-1, n=615; PSUMMIT-2, n=312) were randomised to ustekinumab 45 mg, 90 mg, or placebo, at weeks (wk) 0, 4 and every (q) 12 wks. At wk 16, patients with <5% improvement in tender/swollen joint counts entered blinded early escape. All other placebo patients received ustekinumab 45 mg at wk 24 and wk 28, then q 12 wks. Radiographs of hands/feet at wks 0/24/52 were assessed using PsA-modified van der Heijde-Sharp (vdH-S) scores; combined PSUMMIT-1 and PSUMMIT-2 changes in total vdH-S scores from wk 0 to wk 24 comprised the prespecified primary radiographic analysis. Treatment effects were assessed using analysis of variance on van der Waerden normal scores (factors=treatment, baseline methotrexate usage, and study).
Results Integrated data analysis results indicated that ustekinumab-treated patients (regardless of dose) demonstrated significantly less radiographic progression at wk 24 than did placebo recipients (wk 0–24 total vdH-S score mean changes: 0.4-combined/individual ustekinumab dose groups, 1.0-placebo; all p<0.02). From wk 24 to wk 52, inhibition of radiographic progression was maintained for ustekinumab-treated patients, and progression was substantially reduced among initial placebo recipients who started ustekinumab at wk 16 or wk 24 (wk 24 – wk 52, total vdH-S score mean change: 0.08).
Conclusions Ustekinumab 45 and 90 mg treatments significantly inhibited radiographic progression of joint damage in patients with active PsA.
- Spondyloarthritis
- Anti-TNF
- Psoriatic Arthritis
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
Statistics from Altmetric.com
Introduction
Psoriatic arthritis (PsA) is an inflammatory arthropathy occurring in association with skin psoriasis. The PsA musculoskeletal presentation is heterogeneous, involving peripheral joints, entheses, other periarticular tissues, and/or axial spine. The PsA radiographic spectrum is highly variable and includes patients with mild, non-destructive joint inflammation and those with severe, debilitating joint pain and deformities resulting from progressive joint damage. Diverse radiographic findings are reported to include erosions and joint space narrowing, soft tissue changes, and new bone formation including juxta-articular periosteal reaction and ankylosis.1 ,2 Recent data implicate T helper (Th)-17 cells, key effectors in inflammation and tissue damage in several immune-mediated diseases, as playing a role in the immunopathophysiology of PsA.3 This cytokine axis can be targeted via inhibiting blockade of IL-23, which is required for Th17 cell development, or blocking the activity of the primary cytokine produced by these cells, IL-17.
Reflecting the considerable unmet clinical need, several novel therapeutics have been explored in PsA. One agent, ustekinumab (Stelara, Janssen Biotech Inc, Horsham, Pennsylvania, USA), is a human immunoglobulin G1κ monoclonal antibody that binds to the common p40-subunit shared by IL-12 and IL-23. Ustekinumab is approved for treating moderate to severe psoriasis, as established in large phase three trials.4–6 Ustekinumab also demonstrated efficacy in patients with active PsA in a phase two trial7 and in the larger, phase 3 PSUMMIT-18 and PSUMMIT-29 trials, and has recently gained approval in this indication in the USA and the EU. PSUMMIT-1 included only patients naive to biologic antitumour necrosis factor-α (anti-TNF) treatments, whereas PSUMMIT-2 enrolled patients naive to and previously treated with biologic anti-TNF agents. Change in radiographic progression from baseline at wk 24 using data combined from the two studies, assessed via PsA-modified van der Heijde-Sharp (vdH-S) scores, was a prespecified major secondary study endpoint for both studies. This approach was taken because of the similar trial designs and contemporaneous enrolment and reading of radiographs adopted in the programme, and because the initial power calculation indicated the need for a sample size larger than would be available from either study individually to detect a significant treatment effect (also see online supplementary text). These integrated radiographic analyses through 1 year of ustekinumab treatment in the combined phase 3 PSUMMIT-1 and PSUMMIT-2 trials are reported herein.
Methods
Patients and trial designs
Patient inclusion criteria and trial designs, which were similar across the PSUMMIT-18 and PSUMMIT-29 trials, have been detailed. Briefly, adult patients with active PsA for ≥6 months, despite ≥3 months of disease-modifying antirheumatic agents and/or ≥4 wks of non-steroidal anti-inflammatory agents were eligible. In PSUMMIT-2, 150 to 180 of the 300 planned randomised patients were required to have been previously treated, without consideration of reasons why therapy was discontinued, with biologic anti-TNF agents for at least 8 (etanercept, adalimumab, golimumab, certolizumab) or 14 (infliximab) wks at typical doses. However, entry with less exposure was permitted if there was documented intolerance to a TNF-inhibitor. For both PSUMMIT-1 and PSUMMIT-2, active PsA was defined by the presence of ≥5/66 swollen and ≥5/68 tender joints at screening and baseline, a serum C-reactive protein level ≥6.0 mg/L (modified to ≥3.0 mg/L after study start; upper limit of normal 10 mg/L) at screening, and active or a documented history of plaque psoriasis.
The PSUMMIT-1 (NCT01009086, EudraCT 2009-012264-14) and PSUMMIT-2 (NCT01077362, EudraCT 2009-012265-60) studies were conducted according to the Declaration of Helsinki and International Committee on Harmonisation good clinical practices. The protocols were reviewed and approved by each site's governing institutional review board/ethics committee, reflecting national requirements for study conduct approval. All patients provided written informed consent.
In these phase 3, multicenter, placebo-controlled trials, patients were randomly assigned (1:1:1) to receive ustekinumab 45 mg, 90 mg, or placebo, at wk 0, wk 4 and every 12 wks (q12wks) thereafter. Randomisation was stratified by investigational site, baseline weight (≤/>100 kg), and baseline methotrexate (MTX) usage (yes/no). At wk 16, patients with <5% improvement from baseline in tender and swollen joint counts entered blinded early escape; patients receiving placebo switched to ustekinumab 45 mg, those receiving ustekinumab 45 mg increased to 90 mg, and patients receiving ustekinumab 90 mg continued with their blinded dose regimen. Placebo patients who did not early escape crossed over to receive ustekinumab 45 mg at wk 24, wk 28 and q12wks thereafter.
Radiographic assessments
Radiographic evaluations of the hands and feet were performed at baseline, wk 24 and wk 52 regardless of early escape status, or at the time of study drug discontinuation (unless radiographs were obtained within the prior 8 wks). Centrally digitised images for each patient at all three time points within each trial were scored at the same reading session by two independent readers (and by an adjudicator if the absolute difference of the change from baseline in total vdH-S score between the two readers was >10 or if the change from baseline in total score was missing for one reader), in a random order and without knowledge of time point, patient identity, or treatment assignment; scoring occurred continuously from June 2011 to November 2012 within the clinical programmes. The images were scored using the PsA-modified vdH-S method.10 To assess PsA-specific radiological damage, scores for the distal interphalangeal hand joints and pencil-in-cup/gross osteolysis deformities were added to the original vdH-S score.11 The total PsA-modified radiographic score ranges from 0 to 528 (total score is sum of erosion score (0–320) and joint space narrowing (JSN) score (0–208)). Higher scores and more positive score changes indicate more existing radiographic damage and more radiographic progression, respectively.
Statistical analysis
Progression of structural damage was measured by change from baseline in total PsA-modified vdH-S scores of the hands and feet at wk 24. As prespecified, this major secondary study endpoint was analysed based on an integrated data analysis combining data from the PSUMMIT-1 and PSUMMIT-2 studies with all randomised patients included. Patients were analysed according to randomised treatment group, irrespective of early escape. Analysis of variance on the van der Waerden normal scores, that is, rank-transformed scores based on normal distribution,12 with treatment, patients’ baseline MTX usage (yes/no), and study indicator (PSUMMIT-1 or PSUMMIT-2) as factors in the model was employed to test the treatment difference. To control for multiplicity, the initial analysis tested the difference between the combined ustekinumab groups (45 mg and 90 mg combined) and the placebo group. If this was significant, then pairwise tests, using integrated data from both studies, were to be performed for each individual dose group versus the placebo group.
If the total PsA-modified vdH-S score was missing for a given patient at any of the wk 0, wk 24, or wk 52 visits, missing data were imputed. Two methods were used for imputation. If the X-ray data were available at two time points during the period from wk 0 to wk 24, or from wk 24 to wk 52, the total PsA-modified vdH-S scores at these two time points were linearly extrapolated to the missing visit in the same period. Alternatively, if radiographic data were insufficient for linear extrapolation, the median of the change in the total scores based on all patients within the same MTX stratification at the missing visit was assigned. Please see online supplementary text for a description of the sensitivity analyses performed.
Radiographic data were also analysed as a binary variable based on observed data (no imputation of missing data), using the following cut-points of change from baseline to wk 24 in total vdH-S score for ‘nonprogressors,’ as defined by (1) change less than the smallest detectable change (SDC), which measures the variability of the difference in the change score between two readers, and can be viewed as the amount of change from baseline for which any smaller change cannot be reliably distinguished from random error in the measurement13 and (2) change ≤0.0. The Cochran–Mantel–Haenszel test adjusting for baseline MTX use and individual studies was used to assess differences between the active treatment and placebo groups. All tests were two-sided at a significance level of α=0.05, and radiographic data from the individual studies were analysed in a similar manner. Please see online supplemental text for details of sample size estimation.
Results
Patient disposition and baseline radiographic characteristics
Patient screening began in November 2009, and the wk 52 clinical database locks occurred in July (PSUMMIT-1) and December (PSUMMIT-2) of 2012; the radiographic database was locked in January 2013. Overall, 1771 patients were screened for the PSUMMIT-1 and PSUMMIT-2 trials, of whom 927 patients (747 anti-TNF-naive, 180 anti-TNF-experienced) were randomised to treatment. A majority of the anti-TNF-experienced subgroup had been exposed to ≥2 anti-TNF agents (55% of patients) and had previously demonstrated lack of efficacy or intolerance to anti-TNF-therapy (>70% of patients). The trials were conducted at 189 sites in European (565 patients), North American (319 patients), and Asia-Pacific (43 patients) countries. Patient disposition through 1 year has been previously described for each trial.8 ,9
Missing data were imputed via linear extrapolation or assignment of the median of change scores within the same MTX stratification. For the integrated dataset, the overall rates of missing radiographic data at wk 24 were low in all groups (ie, 2.3–10.3%) and would not be expected to impact the interpretability of the integrated analyses of the effect of ustekinumab on structural damage. Across both studies, median imputation was the predominant means of replacing missing data. However, the PSUMMIT-1 and PSUMMIT-2 studies differed in terms of the amount and pattern of missing radiographic data. The highest rate of missing data was observed in the anti-TNF-experienced population in the PSUMMIT-2 study, mainly resulting from early (within initial 8 wks) study agent discontinuation, and was more than twofold higher among placebo-treated than ustekinumab-treated patients for anti-TNF-experienced (24.2% vs 9.3%) and anti-TNF-naive (11.9% vs 5.6%) patients within the PSUMMIT-2 study. The missing data rate was generally lower and more consistent across treatment groups in the PSUMMIT-1 study (table 1). In all dosing arms, the median changes from baseline in total vdH-S scores and subscores were zero (see table 2). Therefore, the calculations assumed no change in radiographic progression for patients requiring median imputation.
Patients requiring missing data imputation* for change from baseline in total PsA-modified vdH-S score at wk 24; randomised patients in PSUMMIT-1 and PSUMMIT-2
Summary of integrated analyses of change from baseline in total PsA-modified vdH-S score at wk 24; randomised patients
Baseline patient and disease characteristics for the individual PSUMMIT-18 and PSUMMIT-29 trials have been reported in detail. Within each study, approximately 50% of patients were using concomitant MTX at baseline.8 ,9 Baseline vdH-S scores, pooled across both studies, were comparable across the three randomised treatment groups. Baseline median total vdH-S scores were higher in PSUMMIT-1 than in PSUMMIT-2, largely due to numerically lower scores in the PSUMMIT-2 anti-TNF-experienced patients (see online supplementary table S1).
Primary radiographic analysis: integrated data analysis of change from baseline to wk 24 in the total vdH-S score (major secondary study endpoint)
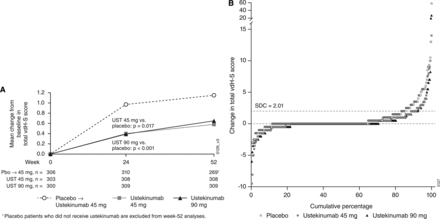
The study achieved the prespecified major secondary endpoint, that is, integrated analysis of change in the total modified vdH-S score at wk 24, such that patients in both ustekinumab dose groups demonstrated significantly less radiographic progression at wk 24 versus patients in the placebo group (table 2, figures 1 and 2). Results of all sensitivity analyses conducted (see online supplementary text) support the robustness of these findings (data not shown). Inhibition of radiographic progression was observed for ustekinumab doses versus placebo, regardless of concomitant MTX status at baseline. In the ∼75% of patients weighing ≤100 kg, less radiographic progression was observed in ustekinumab-treated than placebo-treated patients; no treatment effect was observed in patients weighing >100 kg, although the number of patients in this subgroup was smaller, and the magnitude of radiographic progression was low in the placebo group.
Changes in PsA-modified vdH-S score—mean change from baseline over time through week 52 (A) and cumulative probability plot of change from baseline to week 24 (B). Pbo, placebo; PsA, psoriatic arthritis; SDC, smallest detectable change; UST, ustekinumab; vdH-S, van der Heijde-Sharp.
Changes in PsA-modified vdH-S score from baseline to week 24 for the combined PSUMMIT-1 and 2 ((A)—primary analysis), PSUMMIT-1 (B), and PSUMMIT-2 (C) trials. PsA, psoriatic arthritis; UST, ustekinumab; vdH-S, van der Heijde-Sharp.
As shown in online supplementary table S2 and figure 2, discrete study results indicated that the treatment effect observed in the integrated data analysis was derived from the PSUMMIT-1 study (615 anti-TNF-naive patients), while no clear treatment effect was observed in the smaller PSUMMIT-2 study (312 patients, including 122 anti-TNF-naive and 180 anti-TNF-experienced patients).
Integrated data analysis of additional radiographic endpoints at wk 24
Non-progression of structural damage. At wk 24, significantly higher proportions of ustekinumab-treated (91.7%) than placebo-treated (83.8%; p=0.005 vs combined ustekinumab) patients demonstrated no radiographic progression, as defined by change in total PsA-modified vdH-S score from baseline ≤SDC (=2.01). Similar response patterns were observed when patients were classified as ‘nonprogressors’ based on changes from baseline to wk 24 ≤0.0, although statistical significance was reached only with the 90 mg dose (table 2).
Type of damage. Ustekinumab-treated patients demonstrated significantly less erosive progression than placebo-treated patients (mean changes of 0.2 for 45 and 90 mg vs 0.6 for placebo, p<0.01 for both comparisons). Numerically less JSN was also observed at wk24 in the ustekinumab groups (mean changes of 0.2 for 45 mg and 90 mg) than in the placebo group (mean change of 0.4; p=not significant), and consistent results were observed when change in vdH-S score at wk 24 was analysed for hands and feet separately (data not shown).
Pencil-in-cup and gross osteolysis deformities. The proportions of patients with pencil-in-cup or gross osteolysis deformities were low at baseline (2.6% to 3.8% of patients) and remained stable at wk 24 (3.0% to 3.8%; table 2).
Integrated radiographic data analysis through wk 52
Inhibition of structural damage among ustekinumab-treated patients was sustained beyond wk 24 and through wk 52. Specifically, mean changes in total PsA-modified vdH-S scores among patients initially randomised to either ustekinumab 45 mg or 90 mg were 0.2 and 0.3, respectively, from wk 24 to wk 52, compared with mean changes of 0.4 (45 mg) and 0.4 (90 mg) from baseline to wk 24 (table 3, figure 1B). Consistent with ustekinumab's treatment effect, patients initially randomised to placebo who began receiving ustekinumab 45 mg at wk 16 or wk 24 exhibited a marked decrease in radiographic progression from wk 24 to wk 52 (mean change: 0.1) when compared with that from wk 0 to wk 24 (mean change: 1.1). Of note, the difference in the progression of structural damage between ustekinumab-randomised and placebo-randomised patients persisted through wk 52.
Summary of change in total PsA-modified vdH-S score from wk 0 to wk 24, wk 24 to wk 52, and wk 0 to wk 52; integrated data analysis of randomised patients in the PSUMMIT-1 and PSUMMIT-2 studies
Consistent with findings at wk 24, the proportions of patients with pencil-in-cup or gross osteolysis deformities remained low and stable at wk 52, that is, 9/239 (3.8%) placebo patients for both readers and 19/509 (3.7%) and 13/509 (2.6%) combined ustekinumab patients for readers 1 and 2, respectively.
Discussion
Approximately two-thirds of PsA patients will experience progressive joint damage, which is often associated with functional loss and disability.14 Although the time-course of radiographic progression varies widely, within 2 years of PsA onset, 47% of patients manifest ≥1 erosion and after ≥10 years of follow-up 55% develop ≥5 deformed joints.15
While research in rheumatoid arthritis (RA) has identified a strong link between inflammation and subsequent joint damage, the mechanism behind the structural joint damage in PsA is less defined. Recently, though, collective data from in vitro/in vivo experiments point to the role of IL-23 and the Th17 pathway in PsA's bone erosion and dysregulated bone formation. Specifically, osteoclast precursors (cells that differentiate into osteoclasts via osteoclastogenesis) are elevated in the circulation of PsA patients.16 Also, IL-17 can cause cartilage degradation via effects on chondrocytes.17 IL-23, independent of Th17, also plays a direct role in PsA pathology including enthesitis, a prominent feature of PsA. Sherlock et al 18 demonstrated an IL-23R+ entheseal resident lymphocyte cell population that is activated by IL-23, that produces IL-22, and can promote entheseal and periosteal bone formation.
Beyond these preclinical data, however, evidence for a role of this pathway in PsA bone pathogenesis would ideally arise from targeted inhibition of pathway components in clinical trials. Herein we report that ustekinumab 45 and 90 mg afforded significant and sustained inhibition of radiographic progression in patients with active PsA. This supports the roles of IL-23 and the downstream Th17 pathway in the radiographic damage that occurs in most PsA patients.
Within the limitations of cross-trial comparison, the radiographic treatment effect of ustekinumab among anti-TNF-naive patients in PSUMMIT-1 reported herein appears consistent with those reported for anti-TNF agents in anti-TNF-naive patients with PsA.19–22 This is particularly true when considering the low progression rate among ustekinumab-treated anti-TNF-naive patients in PSUMMIT-1, and when considering the progression observed in placebo-treated patients as a frame of reference. It will, however, be important to monitor the radiographic progression through 2 years of ustekinumab therapy in the PSUMMIT-1 trial to confirm these findings. The observation of less progression of structural damage in obese patients has been reported in RA patients, although the mechanistic basis of this observation has not been determined.23 ,24
Analysis of individual study results indicated the treatment effect observed in the predefined integrated PSUMMIT-1 and PSUMMIT-2 analyses was derived from the PSUMMIT-1 study (see online supplementary table S2). While differences in treatment effect between the two studies may derive from the smaller size and heterogeneity of the PSUMMIT-2 population, the two studies also differed in terms of missing data. The highest rate was observed in anti-TNF-experienced, placebo-treated patients within the PSUMMIT-2 study that included substantial numbers of anti-TNF-refractory patients, which then translated into higher rates of mandated data extrapolation. The higher rate of missing data in PSUMMIT-2 (table 1) was mainly a result of early (within the first 8 wks) discontinuations from the trial, with the majority occurring in the placebo arm. Per protocol, such patients did not qualify for radiographs upon discontinuation due to concerns related to excessive short-term radiation exposure. By contrast, the rate of missing data was generally low and consistent across the treatment groups in PSUMMIT-1 (table 1). Thus, the application of data-handling rules impacted PSUMMIT-2 more than PSUMMIT-1. Specifically, since most missing data required median imputation, the prespecified median imputation rules can be considered to have conservatively favoured the placebo group due to a higher proportion of placebo patients having a score of 0 applied to missing data. Although the treatment effect was not demonstrated in the anti-TNF-naive population in PSUMMIT-2 (unlike PSUMMIT-1), comparison of radiographic findings between these two trial populations is limited by the low progression rate seen across all randomised groups (including placebo) of the PSUMMIT-2 trial relative to placebo-treated patients in PSUMMIT-1.
Thus, ustekinumab 45 mg and 90 mg through 1 year afforded significant and sustained inhibition of radiographic progression in active PsA, supporting the role of IL-23 and the Th17 pathway in the radiographic damage of PsA. This is the first alternate mechanism of action for which the effect on PsA radiographic progression has been established, following anti-TNF agents. The PSUMMIT-1 trial continues through 2 years, and radiographic progression through 2 years of ustekinumab therapy will be assessed. The effect of ustekinumab on progression of structural damage in anti-TNF-experienced patients has not been established, although it also has not been adequately studied.
Acknowledgments
The authors thank Michelle Perate MS, a paid consultant for Janssen Biotech, Inc., and Mary Whitman PhD, an employee of Janssen Biotech, Inc., for writing and editorial support; Lisa T Dooley PhD, an employee of Janssen Research & Development, LLC, for statistical support; and Bruce Randazzo MD PhD and Philippe Szapary MD, employees of Janssen Research & Development, LLC, for assistance with critical review of the manuscript contents. The authors also thank Mittie K Doyle MD, currently an employee of Alexion Pharmaceuticals, for her role in executing the PSUMMIT-1 and PSUMMIT-2 trials, as well as the PSUMMIT-18 and PSUMMIT-29 study investigators.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online supplement
Footnotes
Handling editor Tore K Kvien
-
Collaborators on behalf of PSUMMIT-1 & PSUMMIT-2 Study groups.
Contributors
-
Funding Janssen Research & Development, LLC. (Spring House, PA) provided funding for the studies contributing data to the reported analyses.
-
Competing interests IBM has received grant funding and honoraria from Abbott, BMS, Janssen, Pfizer, Roche, Merck/Schering-Plough, and UCB. PR has received research grant funding and honoraria from Abbott, Amgen, Janssen, Merck/Schering-Plough, and Wyeth. AK has received funding for clinical research sponsored by Abbott, Amgen, Janssen, and UCB. ABG currently has consulting/advisory board agreements in place with Abbott (AbbVie), Actelion, Akros, Amgen, Astellas, Beiersdorf, Biotherapies for Life, Bristol-Myers-Squibb, Canfite, Catabasis, Celgene, Coronado, CSL Behring Dermipsor, GlaxoSmithKline, Incyte, Janssen, Karyopharm, Lilly, Merck, Novartis, Novo Nordisk, Pfizer, TEVA, UCB, Vertex, and Xenoport, and has received research/educational grants (paid to Tufts Medical Center) from Abbott (AbbVie), Amgen, Celgene, Coronado, Janssen, Levia, Lilly, Novartis, and Pfizer. LP has received funding for clinical research and/or honoraria from Abbott, Amgen, Celgene, Janssen, Merck/Schering-Plough, and Pfizer. CR has received research grant support from Janssen and UCB. He has received honoraria from Abbott, Amgen, Janssen, Regeneron, and UCB. SL, YW, LN, CB, MS, and AM are employees of Janssen Research & Development, LLC, the sponsor of the PSUMMIT-1 and PSUMMIT-2 trials.
-
Ethics approval Studies were conducted according to the Declaration of Helsinki and International Committee on Harmonisation good clinical practices. The protocols were reviewed and approved by each site's governing institutional review board or ethics committee, reflecting national requirements for study conduct approval. All patients provided written informed consent.
-
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Editorial
- Clinical and epidemiological research