Article Text
Abstract
Objective: To test the efficacy of standardised monitoring using the disease activity index DAS28 versus usual care on disease modifying antirheumatic drug (DMARD) prescription and disease activity in rheumatoid arthritis.
Methods: A 24 week cluster randomised trial. Rheumatology outpatient centres were randomised to systematic monitoring of disease activity using the DAS28 (12 centres, 205 patients) or usual care (12 centres, 179 patients). The aim for the DAS group was to reach a DAS28 score of ⩽3.2 by changes in DMARD treatment, at the discretion of the rheumatologist and the patient.
Results: At baseline, disease activity was the same in both groups, with an overall mean (SD) DAS28 of 4.5 (1.2); 13% of the patients had a DAS28 of ⩽3.2. At 24 weeks, 31% of patients in the DAS group had a DAS28 ⩽3.2, while in the usual care centres this was 16% (p = 0.028). DMARDs were changed on average in 18% of visits in the DAS centres; in the 12 usual care centres they were changed on 8% of the visits (p = 0.013). The doses of methotrexate, sulfasalazine, and corticosteroids appeared to be higher in the DAS centres than in the usual care centres, but the differences were not significant.
Conclusions: In daily practice, systematic monitoring of disease activity in rheumatoid arthritis may lead to more changes in DMARD treatment, resulting in a larger number of patients with low disease activity.
- DAS28, 28 joint disease activity score
- DMARD, disease modifying antirheumatic drug
- RCT, randomised controlled trial
- UC, usual care
- rheumatoid arthritis
- disease activity
- decision support
Statistics from Altmetric.com
- DAS28, 28 joint disease activity score
- DMARD, disease modifying antirheumatic drug
- RCT, randomised controlled trial
- UC, usual care
For the management of rheumatoid arthritis, there is general agreement that rheumatoid inflammation should be controlled as soon as possible, as completely as possible, and for as long as possible, consistent with patient safety.1,2 When the goal of treatment is to reach optimal control of rheumatoid inflammation, it is clear that this should be evaluated systematically. Subsequently, the treatment programme can be adjusted from the perspective of both benefit and harm.3 The combination of systematic evaluation and clinical guidelines could provide valuable decision support for optimising the management of rheumatoid arthritis. The effects of such decision support should preferably be studied using a randomised controlled trial design (RCT).4 However, when studying the effects of decision support (such as guidelines) in a trial, it is generally not necessary to study the effect on health5 because, if the guidelines are based on sound evidence, it is already known that the targeted behaviour will be beneficial. For example, the “efficacy” of guidelines to improve folate supplementation in addition to methotrexate can be evaluated simply by counting the number of correct prescriptions. However, in the case of guidelines for the control of rheumatoid inflammation in rheumatoid arthritis, physician performance—as reflected by disease modifying anti-rheumatic drug (DMARD) prescription—is more difficult to judge: prescribing more DMARDs is not identical to better treatment. Thus the proportion of patients with adequately controlled rheumatoid inflammation (for example with a 28 joint disease activity score (DAS28) of ⩽3.2) can be proposed as a proxy for physician performance in such trials.4 Having a low level of rheumatoid inflammation (DAS28 ⩽3.2) over time is associated with up to 50% less progression of joint damage.6 Also, functional capacity (as judged by the health assessment questionnaire (HAQ)) is predominantly determined by rheumatoid inflammation (disease activity score (DAS)) early in the disease, and by joint damage later.7 The advantages of using the DAS288,9 for the assessment of rheumatoid inflammation in daily practice are as follows: first, the DAS28 is more valid for measuring underlying rheumatoid inflammation than individual indices of inflammation; second, the continuous scale of the DAS28 has absolute meaning, making its value interpretable, unlike a measure of percentage change; third, low disease activity as reflected by a DAS28 of ⩽3.2 reflects a clinical meaningful target for DMARD treatment; and fourth, the DAS is also used in clinical trials, which makes it easier to translate trial results into clinical practice.
The objective of this trial was to test the efficacy of standardised monitoring using the DAS28 versus usual care on DMARD prescription and disease activity in rheumatoid arthritis.
METHODS
This study was a multicentre, 24 week, cluster randomised controlled trial of systematic monitoring using the DAS28 (DAS group) versus usual care (UC group) in rheumatoid arthritis. In addition, all patients included started using celecoxib, a COX-2 specific inhibitor, 200 mg twice daily. The results of the celecoxib use will be reported separately.
The primary end points of this study were:
-
the proportion of patients reaching low disease activity (DAS28 ⩽3.2) at week 24 in a subgroup of patients;
-
the changes in DMARD treatment during 24 weeks, in all patients.
Secondary end points were the dose changes in individual DMARDs and changes in patient assessed pain, global disease activity, and disability.
To determine the proportion of patients with a DAS28 ⩽3.2 in this trial, the DAS28 had to be assessed independently. For reasons of efficiency, these independent assessments only took place in a subgroup of patients, consisting of all patients from the participating centres in a predetermined geographical region.
Sample
Twenty four rheumatology outpatient centres throughout the Netherlands were willing to participate in the study. A statistician used a random number generator to allocate the centres randomly to DAS (12 centres) or UC (12 centres). All patients within a centre were treated in the same way. Randomisation took place in two strata: one stratum consisted of the participating centres in the predetermined region; the other consisted of all other participating centres. Centre allocation remained concealed until the start of patient recruitment. The period of recruitment began in March 2000 and ended in March 2001. Patients with rheumatoid arthritis who were in need of NSAID treatment were asked by their treating rheumatologist to participate. All patients included started treatment with celecoxib 200 mg twice daily.
Inclusion criteria were: outpatients of at least 18 years of age with rheumatoid arthritis according to the ACR criteria; medical need for NSAID treatment; adequate anticonception measures; and provision of informed consent.
Exclusion criteria were: a history of allergy to NSAIDs; serious bowel, liver, kidney, or heart disease; coagulopathy; (suspicion of) peptic ulcer or gastrointestinal bleeding; malignancy; and substance abuse or mental disorders that would interfere with study participation.
Interventions
In the DAS group, systematic monitoring of disease activity was carried out at week 0, 4, 12, and 24 by assessment of the DAS28 by the treating rheumatologist. According to the study guidelines, the aim was to reach a DAS28 ⩽3.2 (low disease activity) by changing DMARD treatment if the score was above 3.2.9 The rheumatologists of the DAS group had been instructed in performing the joint counts and in using a special calculator for the DAS28.
In the UC group, no systematic monitoring of disease activity was done and no guideline to adapt treatment strategy was supplied. Otherwise, the study visits were identical in both groups.
Assessments
Registration of past and current drug treatment use took place at 0, 4, 12, and 24 weeks. A stop, start, or addition of a DMARD, or a change in DMARD dose, was considered to be a change in DMARD treatment.
The DAS28 was calculated according to the formula of Prevoo et al.8 The DAS28 includes a 28 tender joint count, a 28 swollen joint count, erythrocyte sedimentation rate (ESR), and a general health item (GH). ESR was determined locally using the Westergren method. GH was rated by the patient on a numerical rating scale of 0 (very well) to 10 (very bad), which was rescaled to range from 0 to 100.
Patient assessed pain and global disease activity were also rated on numerical rating scales from 0 to 10. A validated Dutch version of the disability index of the Stanford HAQ was used to assess disability; the HAQ ranges from 0 to 3.10
In addition, the joint counts for the DAS28 were independently assessed at weeks 0 and 24, in the three DAS and the four UC centres that were in the predetermined geographical region. Specially trained research nurses carried out these joint counts. The rheumatologists did not have access to the results of these assessments.
Statistical analysis
The data were analysed using linear regression with random coefficients (mixed models), correcting for clustering of the data in centres (all analyses) and additionally correcting for repeated measurements (analysis of DMARD changes). An intention to treat approach with last observation carried forward was used for the analysis of primary outcomes. Probability (p) values of <0.05 were considered statistically significant.
In addition, the agreement between the DAS28 as assessed by the rheumatologists and the independent research nurses was analysed using an intraclass correlation coefficient (ICC2,1) for agreement. The analyses were carried out using the SAS 8.0 statistical software package.
Sample size determination
The sample size needed was calculated for detecting differences in disease activity in the subgroup. The expected mean (SD) change in DAS28 in the DAS group was −1.0 (1.0) and in the UC group −0.5 (1.0). With α = 0.05 and 1-β = 0.90 it would need 2×85 = 170 patients. In that case, a 16% difference in the proportion of patients with DAS28 ⩽3.2 would be significant if the mean (SD) DAS28 at baseline was 4.2 (1.2), which is a usual mean DAS28 for samples from the clinical rheumatoid population. It was expected that the degree of clustering, and therefore inflation of sample size, would be low (ICC = 0.005) because of the independent assessment of the DAS28.
RESULTS
Sample
The 12 centres in the DAS group enrolled 205 patients, and the 12 UC centres enrolled 179 patients. Patients of both groups were comparable at baseline (table 1) except for rheumatoid factor (RF) positivity (p<0.05 in main sample and subgroup). There were no other statistically significant differences. In the DAS group there were 16 dropouts (8%), for the following reasons: adverse event (n = 3); patient wish (n = 5); other reason (n = 8). In the UC group there were 20 dropouts (11%): adverse event (n = 9); patient wish (n = 7); other reason (n = 4). Dropouts for “other reason” included protocol violations by starting leflunomide (five in the DAS group, two in the UC group) or infliximab (one in DAS group).
Baseline variables for the main study (n = 384) and its subgroup (n = 142)
Disease activity
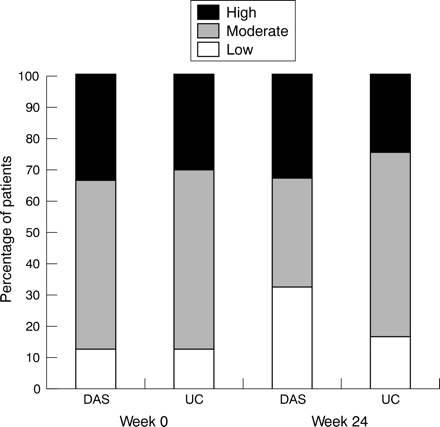
Achieving low disease activity was determined for the subsample only (n = 142). At baseline, the DAS (n = 61) and UC (n = 81) groups had comparable levels of disease activity. The mean (SD) DAS28 at baseline was 4.6 (1.2) in the DAS group and 4.5 (1.2) in the UC group (t test, p = 0.44); eight of 61 patients in the DAS group (13%) had low disease activity (DAS28 ⩽3.2), and 10 of 81 (12%) in the UC group (χ2, p = 0.91).
At 24 weeks, the number of patients with low disease activity in the DAS group increased to 19 (31%), while in the UC group there were 13 (16%) (fig 1). The mean difference (with 95% confidence interval (CI)) in the proportion of patients with low disease activity at week 24 was 15% (3% to 27%) (mixed models; p = 0.028). The mean (SD) changes in DAS28 over 24 weeks were −0.40 (1.0) in the DAS group and −0.14 (1.2) in the UC group (mixed models; p = 0.36). The DAS28 values as assessed by the rheumatologists and the independent research nurses agreed, with ICC = 0.88 at week 0 and 0.89 at week 24.
In the disease activity score (DAS) group, more patients reached low disease activity (DAS28 ⩽3.2) than in the usual care (UC) group (p = 0.028).
DMARD changes
At baseline, methotrexate and sulfasalazine were the most often used DMARDs (table 2). There were no significant baseline differences between the two groups. In the DAS centres, there were more DMARD changes during the study (fig 2). DMARD changes took place on average in 20% of visits to the DAS centres and in 9% to the UC centres. The corrected mean difference (95% CI) was 9% (2% to 16%) (mixed models; p = 0.013).
Disease modifying antirheumatic drug, corticosteroid, and non-steroidal anti-inflammatory drug use at the start of the study
In the disease activity score (DAS) group, more changes in disease modifying antirheumatic drug (DMARD) treatment occurred during the course of the study (p = 0.013).
Drug use
To provide insight into the nature of changes in drug treatment, the mean dose of the three most frequently used drugs is shown in fig 3, panels A to C. Seen graphically, it appeared that dosages were higher in the DAS group. However, there were no statistically significant differences between the DAS and UC groups in mean methotrexate dose (mixed models; p = 0.11), mean SSZ dose (mixed models; p = 0.27), and mean prednisone dose (mixed models; p = 0.29). There also appeared to be no large differences in the amount of non-oral steroids given. Regarding celecoxib use, a small number of patients continuing the study stopped using this drug. In the DAS group 21 of 205 (10%) stopped using celecoxib (nine because of adverse events), and in the UC group 22 of 179 (12%) stopped using it (13 because of adverse events).
Mean dose of methotrexate (A), sulfasalazine (B), and oral prednisone (C) during the course of the study.
Patient assessments
At baseline, there were no significant differences in assessments of pain, global disease activity, and disability between the patients of the DAS and UC groups. The improvements in patient assessments appeared to be larger in the DAS group than in the UC group, but there was a significant difference in patient global assessment of disease activity only (not shown).
Adverse events
The most frequently occurring adverse events were: upper abdominal pain, 50/384 (13%); infections, 42/384 (11%); rash or itching, 29/384 (8%); nausea or vomiting, 24/384 (6%); and headaches, 18/384 (5%). There were significant differences (p<0.05) only for rash or itching (DAS group 4%; UC group 11%) and nausea or vomiting (DAS group 4%; UC group 9%). There appeared to be no tendency for adverse events to occur more often in the DAS group.
Adherence to the intervention
In the DAS group, the DAS28 was calculated by the rheumatologists on 99% of the visits, and 93% of the DMARD changes occurred when disease activity was moderate or high. However, in all the instances where the DAS28 exceeded 3.2, a DMARD change took place on average in only 20%. The most frequently mentioned reasons for not changing DMARDs when the DAS28 was above 3.2 were “wait and see” and “disease activity is assessed as sufficiently low” (table 3).
Reasons of rheumatologists not changing drug treatment at visits where the DAS28 exceeded 3.2, given by 93 of the DAS patients
DISCUSSION
This multicentre cluster RCT of standardised monitoring of disease activity versus usual care showed that standardised monitoring resulted in more changes in DMARD treatment during the 24 week study period, and in twice as many patients with a low disease activity at 24 weeks. We conclude that standardised monitoring of disease activity in rheumatoid arthritis with the aim of achieving low disease activity improves the management of rheumatoid patients in daily clinical practice.
There are several ways in which standardised monitoring may be useful in clinical practice: monitoring can be used to measure and document treatment need; it can support the use of specific DMARDs; it can ensure that rheumatoid inflammation is still under control; it is useful for assessing whether the treatment chosen is necessary and effective; and it can ensure that no overtreatment is given.2 Apart from assessing disease activity, monitoring may include assessments for disability and joint damage.11 At the same time, it must be clear that, while standardised measures can support clinical decision making, they do not replace careful patient examination and inquiry.11 Experienced rheumatologists may make risk–benefit assessments and decisions about treatment (with their patients) that are hard to capture or standardise with standard measures.
In this study, we chose to add only the evaluation of rheumatoid inflammation to usual care, because rheumatoid inflammation is the primary target of DMARD treatment. Although we found a relevant and significant difference in the proportions of patients with low disease activity (DAS28 ⩽3.2) at 24 weeks, the decrease in rheumatoid inflammation was smaller than expected, and was significant only in the difference in proportions of patients with DAS28 ⩽3.2, but not in the difference in continuous DAS28. The latter is unexpected, as usually power is lost when dichotomising a continuous measure, but it may be explained by the DAS28 ⩽3.2 being a more direct reflection of the study guidelines provided. The relatively small difference between the DAS group and UC group probably reflects freedom of choice of treatment options within the study guidelines, and the start of a new drug (celecoxib) at baseline. Both may have led to less DMARD change in the DAS group than might otherwise have occurred. Of note, in 80% of instances with a DAS28 >3.2 in the DAS group, the DMARD treatment remained unchanged. The reasons for not changing DMARDs given by the physicians indicated that the level of inflammation reached was often judged satisfactory. However, at DAS28 values above 3.2, rheumatoid inflammation is certainly not sufficiently controlled.6,9 This discrepancy between satisfaction and objective level of rheumatoid inflammation may require more attention in future studies and implementation initiatives. In our experience, physicians and patients are often satisfied with treatment because of a decrease in inflammation. However, the goal of treatment is not so much to induce a decrease, but to keep inflammation under control, for which further adaptations in DMARD treatment may be necessary.2,3
This trial was a cluster randomised trial with the outcome being measured and analysed at patient level (as opposed to analysis at cluster level), correcting for the dependency of patients within clusters by using mixed models. The patient samples were comparable at baseline, particularly in the primary outcome variables of DAS28 and drug use. This is a particular concern in cluster randomised trials. As the participating physicians could not be blinded, it was necessary to measure the DAS28 independently, but unfortunately this could not be done in all the centres. A larger sample would not necessarily have led to different DAS28 changes, but to better precision in the estimation of differences. The number of dropouts in this study was small and comparable in both study groups, and the results of the intention to treat analysis did not differ from the per protocol analysis. For all these reasons, we feel that it is unlikely that the results of our study can be explained by bias in its design.
Evidence on the effects of monitoring of disease activity in rheumatoid arthritis is sparse. Monitoring and guidelines are not interventions that will cause health effects in themselves, but drug treatment may do so. This indirectness makes it difficult to study and detect “health effects” of monitoring and guidelines. There have been two pre-experimental studies finding moderate evidence in favour of monitoring,12,13 and recently another RCT was published.14 In that study, a combination of systematic monitoring of disease activity and a strict protocol of increasing DMARD treatment, compared with usual care, was investigated in patients with active and early rheumatoid arthritis. In 24 weeks the mean DAS28 (as converted from the original DAS9) of the usual care group had decreased from 5.8 to 4.6, but that of the monitoring group had decreased much more, from 6.2 to 3.4. A mean DAS28 of 3.4 means that between 40% and 50% of the patients will have a low disease activity according to the DAS28 (DAS28 ⩽3.2). By comparison, in our study only 31% had such a low DAS28 at 24 weeks. A major difference between these two RCTs is that the intervention by Grigor et al was undertaken by a single rheumatologist who used a strict protocol of escalating DMARD treatment with monthly visits, whereas in our study the choice of DMARD treatment was at the discretion of the treating rheumatologists and visits were less frequent.
These studies provide evidence that in daily practice, systematic monitoring of disease activity in rheumatoid arthritis may lead to more changes in DMARD treatment, resulting in more patients with low disease activity. The stricter use of monitoring and guidelines in targeting DMARD therapy may lead to better results.
Acknowledgments
This study was made possible by Pfizer. We are grateful for the support and critical reading of the manuscript by Sherida Tjon a Hen MA, of Pfizer. We also wish to thank Aggie Smetsers MA for her help in editing this manuscript.
REFERENCES
Footnotes
-
Published Online First 13 April 2005