Abstract
S. pneumoniae is a significant cause of community-acquired pneumonia in the elderly, and accounts for the majority of the pneumonia deaths among the elderly. We conducted this randomized double-blind study to evaluate the immune response to a 23-valent pneumococcal polysaccharide vaccine and the persistence of antibodies two years after the vaccination in an elderly population in Santiago, Chile. A total of 118 elderly nursing home residents received either the pneumococcal or a tetanus control vaccine. Serum samples were taken at enrolment, at two months, and at two years post-vaccination. Pre-vaccination anti-pneumococcal antibody geometric mean concentrations (GMC) were similar in both study groups, with increased levels of antibodies found only against serotype 14. The pneumococcal vaccine was highly immunogenic at 2 months, and titers remained high two years after the vaccination for the 10 serotypes studied in this elderly population. The results thus support the benefits of this pneumococcal vaccine in this elderly population who are at increased risk of invasive pneumococcal disease.
Streptococcus pneumoniae; vaccine; elderly; immunogenicity; antibodies
ORIGINAL PAPERS
Immunogenicity of a 23-valent pneumococcal polysaccharide vaccine in elderly residents of a long-term care facility
M. Teresa Valenzuela B.I; Rogelio Altuzarra H.II; Olivia Trucco A.II; Rodrigo Villegas R.I; Jaime Inostroza S.III; Paulo Granata S.II; José Fleiderman V.II; Leonardo Maggi C.II
IUniversity of los Andes and Medical School of Public Health, University of Chile
IIMedical School of University of los Andes
IIIDepartment of Basic Sciences, Medical School of University of La Frontera; Santiago, Chile
Address for correspondenceAddress for correspondence: Dr. M. Teresa Valenzuela Facultad de Medicina Universidad de los Andes San Carlos de Apoquindo 2200, Edificio Biblioteca, Tercer Piso, Las Condes, Santiago Phone: (56-2) 4129477. Fax: (56-2) 2141752 E-mail: mtvalenzuela@uandes.cl
ABSTRACT
S. pneumoniae is a significant cause of community-acquired pneumonia in the elderly, and accounts for the majority of the pneumonia deaths among the elderly. We conducted this randomized double-blind study to evaluate the immune response to a 23-valent pneumococcal polysaccharide vaccine and the persistence of antibodies two years after the vaccination in an elderly population in Santiago, Chile. A total of 118 elderly nursing home residents received either the pneumococcal or a tetanus control vaccine. Serum samples were taken at enrolment, at two months, and at two years post-vaccination. Pre-vaccination anti-pneumococcal antibody geometric mean concentrations (GMC) were similar in both study groups, with increased levels of antibodies found only against serotype 14. The pneumococcal vaccine was highly immunogenic at 2 months, and titers remained high two years after the vaccination for the 10 serotypes studied in this elderly population. The results thus support the benefits of this pneumococcal vaccine in this elderly population who are at increased risk of invasive pneumococcal disease.
Key-Words: Streptococcus pneumoniae, vaccine, elderly, immunogenicity, antibodies.
Streptococcus pneumoniae can result in conditions ranging from nasopharyngeal colonization to otitis media, and invasive diseases such as meningitis, sepsis, bacteremia, or pneumonia. S. pneumoniae may account for 30% to 50% of all adult community-acquired pneumonias (CAP) requiring hospital admission and may also be the most frequent cause of pneumonia in long term care institutions [1,2]. A meta-analysis of 122 reports of CAP between 1966 and 1995 showed that S. pneumoniae caused 66% of nearly 7,000 cases having an established etiology [3].
The reported annual incidence of pneumococcal infections worldwide is between 130 and 210 cases for every 100,000 inhabitants, but the rate is higher in elderly persons and in patients with a compromised immune system [4]. Recent data suggest that the rate of CAP in the US is 18.2 cases per 1,000 person-years in persons aged 65 to 69 years, rises to 52.3 cases per 1,000 person-years in those aged 85 years and older, and in nursing home residents, the rates of CAP are estimated to be more than 300 cases per 1,000 person-years [5-7]. The annual incidence of invasive infections due to Streptococcus pneumoniae in adults older than 64 years in Chile, in a follow-up period of five years, has been reported to be 234/100,000 [8]. The mortality rate for pneumonia in 1998 in Chile was 83/100,000 inhabitants [9].
Pneumonia in elderly patients is often caused by microaspiration of upper respiratory tract secretions colonized by S. pneumoniae, especially in those with an alteration in local defense mechanisms. Elderly individuals often have medical histories, underlying conditions, or chronic diseases that increase their risk of pneumonia, such as chronic cardiac and pulmonary conditions, diabetes mellitus, alcoholism with liver failure, dementia, cerebrovascular diseases, and a smoking habit [10,11]. Pneumococcal diseases are traditionally community-acquired pathologies. Long-term care facilities, or nursing homes, are unique among community settings, providing an environment rich in susceptible hosts and ideally suited for spread of respiratory diseases.
Whether the infection is acquired in the community or in long-term care, more than 80% of invasive pneumococcal isolates from older adults are serotypes included in the 23-valent pneumococcal vaccine (Pneumo 23, Sanofi Pasteur, Lyon France) currently available in Chile [8]. This finding highlights the potential benefits expected from immunizing this high-risk population. Our primary objective was to determine the immunogenicity of this 23-valent pneumococcal polysaccharide vaccine in elderly in a long-term care institution at 2 months and at 2 years post-vaccination.
Materials and Methods
Study Design and Population
This double-blind, randomized, controlled, study enrolled subjects who were residents of Hogar de Ancianos Rosita Renard residence for the elderly, between August 1998 and September 2000. The study protocol and consent form were approved by the study center Ethical Review Committee of Universidad de Los Andes, and all subjects gave written informed consent before study entry. Before entering the study, a physician examined each patient and obtained a clinical history. Subjects were randomly assigned in equal numbers to receive a 23-valent pneumococcal polysaccharide (Group B), or a monovalent tetanus toxoid control vaccine (Group A). Male and female residents, 60 years of age or older were eligible if they had a normal immune system, were self sufficient in carrying out daily activities, and were receiving adequate treatment for any chronic disease or condition if present. Patients with an acute febrile illness 7 days prior the study, or immunocompromised subjects with a high risk of pneumococcal disease or complications, spleen dysfunction, splenectomy, lymphoma, multiple myeloma, chronic kidney failure or organ transplant candidates, HIV/AIDS, and individuals with cerebrovascular disease, diabetes mellitus or alcoholism were excluded. Subjects having a pneumococcal vaccination within a period of 6 years were also excluded.
Vaccines
The studied vaccine was Pneumo 23, a polyvalent polysaccharide vaccine. Each 0.5 mL dose contains 25 mg of each of the following 23 polysaccharide serotype antigens: 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14,15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. The control vaccine was TetavaxTM a monovalent tetanus toxoid vaccine. Both vaccines were produced and supplied by Sanofi Pasteur, Lyon, France, and a single dose was administered by intramuscular injection into the delthoid region.
Immunology
A pre-vaccination blood sample was taken from each subject. Two months after vaccination, a second blood sample was obtained to measure the antibody response to 10 pneumococcal serotypes. In order to evaluate antibody persistence, a third blood sample was obtained approximately 2 years after vaccination.
The antibody concentration was determined by ELISA using polysaccharide antigens obtained from the American Type Culture Commission (ATCC, Manassas, Virginia, USA) using a previously described method in which association between the antibody concentration detected by ELISA and opsonophagocytic activity (OPA) was improved [12]. Geometric Mean Concentrations (GMC) and 95% confidence intervals (95% CI) were calculated for anti-pneumococcal antibodies against the following 10 serotypes: 1, 3, 4, 5, 6B, 9V, 14, 18C, 19F and 23F. Descriptive comparisons were made of GMCs based on the calculated 95% CI. Pre-vaccination GMCs >1.3 mg/mL of antibodies, (equivalent to 200 ng IgG/mL) were defined as the threshold seropositive level [13]. Vaccine response/seroconversion was defined a 2-fold increase over pre-vaccination levels in GMCs at 2 months and at 2 years after vaccination.
Reactogenicity and Safety
Reactogenicity and safety were evaluated by subject reports one hour, 24 hours and 48 hours post-immunisation. We recorded local and systemic reactions related to vaccination.
Results
Subject Disposition
From 118 subjects, 55.1% were men and 44.9% women. Chi-square test was used to compare the two groups of study, vaccinated and the control groups. There were no differences in the coexisting diseases of the patients (p > 0.9). Nineteen point five per cent had cardiac disease, 41.5% had hypertension, 9.3% diabetes mellitus, and 10.2% chronic lung disease. The demographic data for the study population is summarized in Table 1. Antibody levels were measured before and 2 months after pneumococcal vaccination in 30 subjects, and a third measurement was made at 2 years in 15 subjects. In the control group, 41 subjects were evaluated before vaccination and two months later; antibody levels were measured in 22 subjects at 2 years. Decreases in the numbers of subjects available between enrollment (n = 118), at 2 months (n = 71), and at 2 years (n = 37) resulted from transfers to other institutions and blood samples that were insufficient for the laboratory analysis. For ethical reasons the investigators decided not to obtain another sample.
Immunology
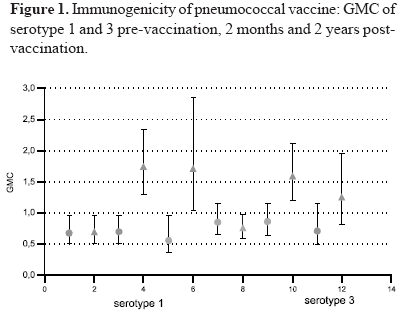
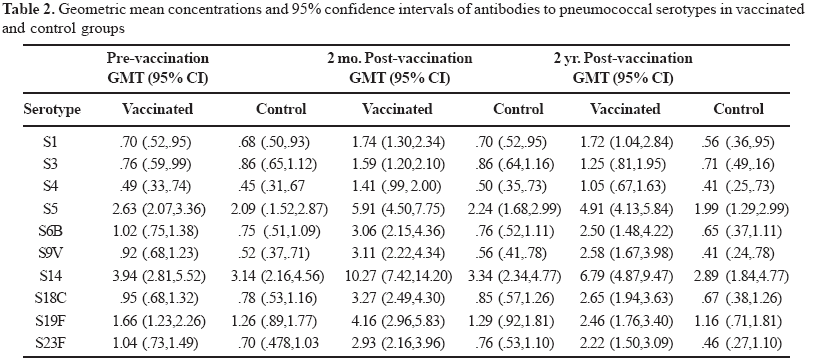
The immunologic data for each of the 10 pneumococcal serotypes tested are listed in Table 2. The highest pre-vaccination titers in both study groups were of antibodies against serotype 14, with values of 3.1 mg/mL. (95% CI:2.2; 4.6) and 3.9 mg/mL. (95% CI:2.8; 5.5) respectively. The lowest pre-vaccination GMCs were those against serotype 4 in both groups with values of 0.45 mg/mL (95% CI: 0.3-0.7) and 0.49 mg/mL (95% CI:0-3-0.7). Before vaccination, the anti-pneumococcal antibody GMCs for the ten serotypes evaluated were similar. The highest GMCs two months after vaccination were against serotypes 14, 5, 19F and 18C in the group receiving pneumococcal vaccine, with values of 10.27 mg/mL (95% CI:7.4-14.2), 5.90 mg/mL (95% CI:4.5-7.7), 4.15 mg/mL (95% CI:2.96-5.8), and 3.27 mg/mL (95% CI:2.5-4.3) respectively. In the subjects vaccinated with the pneumococcal vaccine, the antibody levels for each serotype remained increased two years after vaccination, and the GMCs in this group were higher at 2 months and at 2 years for all the antibodies evaluated than those in the group receiving the control vaccine (Table 2). The pre- and post-vaccination GMCs are shown in Figures 1 to 4.
Reactogenicity and Safety
The most frequently reported local events within 1 hour following pneumococcal vaccination were pain at the injection site (15.3%), and redness (3.4%); 81.3% of subjects did not report any reaction. At 24 hours, 5.9% reported pain at the injection site, 5.9% experienced redness and 0.8% swelling. At 48 hours post-vaccination, only 5.9% of the subjects experienced redness, 1.7% swelling and 1.7% pain. No systemic adverse events were observed.
Discussion
Our objective was to measure the immediate immune response to 10 of the serotypes contained in the vaccine 2 months after vaccination in an elderly population, as well as the persistence of antibodies two years after vaccination. Our results confirmed the high immunogenicity of this vaccine as well as the fact that the antibody levels against the serotypes measured remained high throughout two years of follow-up. High pre-vaccination levels of anti anti-serotype 14 antibodies in the studied population can be explained by the high prevalence of this serotype in colonization or invasive infections in our environment [14,15].
We do not have clear information about the protective antibody levels in adults. However, in children who have not received pneumococcal vaccine, a GMC of at least 1.3 mg/mL for any of the ten serotypes studied appears protective [13]. Pre-vaccination titers in our study group had such increased values only for three serotypes: 5, 14 and 19F. In a second sample, two months after vaccination, there was 100% seroconversion in the serotypes studied, defined as at least a two fold increase in GMC, and all were above the presumed protective concentration.
It is also important to know how long the antibodies remain above a protective level, especially in elderly patients who are at increased risk for pneumococcal diseases. Two years after vaccination, the levels of antibodies were below the presumed protective levels only for serotype 4 (1.05 mg/mL). In a previous study conducted in a nursing home population, a decline in seroconversion rates following vaccination has been seen for serotype 14, and serotypes 6B and 23F have also been less immunogenic in relation to responses to other pneumococcal capsular antigens [14]. In general, our immunogenicity findings are similar to those previously described, which found stronger responses to serotypes 9V and 14 [16]. It is important to emphasize that it is difficult to compare the GMCs in our results with those of earlier studies, since the method for determination of antibody concentration changed in the year 2000 to one in which cross-reactivity between pneumococcal serotypes in the test panels and the subject samples were reduced [12]. We used the new methodology in our study.
Pneumococcal polysaccharide vaccines have been licensed by many countries worldwide since their introduction in 1982, and have been included in programs for prevention of serious respiratory diseases in the elderly, especially pneumonia in developed countries. The recommendation for pneumococcal vaccination in the elderly is often combined with that for influenza vaccination [17]. Data on the efficiency of this vaccine is limited for various reasons, among them, lack of a surveillance system with defined diagnostic and confirmation standards for pneumococcal pneumonia in patients over 65 years old. The available data indicate effectiveness between 48% and 81% for prevention of invasive disease, depending on the study [18-20]. Health economics investigations data support the cost-effectiveness of pneumococcal polysaccharide vaccines in elderly populations [21,22]. The American Advisory Committee on Immunization Practice (ACIP) recommends vaccination of high-risk groups, who are two years of age or older and healthy people 65 years of age or older [23].
The social, economic and medical burden of pneumococcal disease, especially in the elderly, immunocompromised patients, or those with chronic diseases, together with an increase in antibiotic resistant S. pneumoniae, creates a rationale for use of pneumococcal polysaccharide vaccine in these groups. We conclude that the immune response and persistence of antibody concentrations in response to the pneumococcal vaccine observed in this studyprovide important additional support for its routine use in elderly populations, who, like those in our study, are at increased risk of pneumococcal infection.
Acknowledgements
The authors would like to acknowledge the collaboration of the health personnel of Hogar de Ancianos Rosita Renard, Hogar de Cristo. We also acknowledge the contribution made by Sanofi Pasteur, who kindly donated the vaccine for this study.
Received on 5 January 2007; revised 30 May 2007.
Proyectos de Investigación Científica de la Universidad de los Andes. Sanofi Pasteur, Lyon, France.
- 1. High K.P. Pneumonia in older adults New categories add complexity to diagnosis and care. Postgraduate Medicine 2005;118:18-28.
- 2. Fedson D., Anthony J., Scott G. The burden of pneumococcal disease among adults in developed and developing countries: what is and is not known. Vaccine 1999, S11-S8.
- 3. Kalin M., Örtqvist A., Almeda M., et al. Prospective study of prognosis factors in community-acquired bacteremic pneumococcal disease in 5 countries. J Infect Dis 2000;182:840-7.
- 4. Obaro S.K., Monteil M.A., Henderson D.C. The pneumoccocal problem. BMJ 1998;312:1521-5.
- 5. Muder R.R., Aghababian R.V., Loeb M.B., et al. Nursing home-acquired pneumonia: an emergency department treatment algorithm. Curr Med Res Opin 2004;20:1309-20.
- 6. Loeb M. Epidemiology of community- and nursing home-acquired pneumonia in older adults.Expert Rev Anti Infect Ther 2005;Apr;3(2):263-70.
- 7. Jackson M.L., Neuzil K.M., Thompson W.W., et al. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis 2004;39:1642-50.
- 8. Inostroza J., Vinet A., Retamal P., et al. Influence of patient age on Streptococcus pneumoniae serotypes causing invasive disease. Clin Diagn Lab Immunol 2001:8(3):556-9.
-
9MINSAL. División de Salud de las personas. Programa Especial de Control de las Enfermedades Respiratorias del Adulto en Chile, Marzo, 2001
- 10. Fine M.J., Smith M.A., Carson C.A., et al. Prognosis and outcomes of patients with community-adquired pneumonia. JAMA 1996;275:134-41.
- 11. Centers for Disease Control and Prevention. Influenza and pneumococcal vaccination levels among adults aged > 65 years. United States. MMWR Morbid Mortal Wkly Rep 1998;47:797-802.
- 12. Soininen A., Karpala M., Wahlman L., et al. Specifities and opsonophagocytic activities of antibodies to pneumococal capsular polysaccharides in sera of unimmunized young children. Clinical and Diagnostic Laboratory Immunology 2002;5:1032-8.
- 13. Laurence E.M., Edwards K.M., Schiffmann G., et al. Pneumococcal vaccine in normal children. An J Dis Child 1983;137:846-50.
- 14. Altuzarra R., Valenzuela M.T., Trucco O., et al. Portación nasal de Streptococcus pneumoniae en Adulto mayor y su respuesta frente a la vacunación antineumocócic. (accepted for publication).
- 15. Hormazabal J.C., Heitmann I., Rojo P., et al. Serotipos de S.pneumoniae asociados a patología invasiva del adulto. Instituto de Salud Pública, Laboratorio de Referencia cocáceas Gram positivas. Programa Microbiología-Micología ICBM, Facultad de Medicina, Universidad de Chile. Presentado en el XIX Congreso Chileno de Infectología. Nov. 2002
- 16. Butler J., Shapiro E., Carlone G. Pneumococcal vaccines: History, current status and future directions. Am J Med 1999;107(1ª):69S-76S.
- 17. Nichol C.L. The additive benefits of influenza and pneumococcal vaccinations during influenza seasons among elderly persons with chronic lung disease. Vaccine 1999;17:S91-S3.
- 18. Sims R.V., Steinmann W.C., McConville J.H., et al. The clinical effectiveness of pneumococcal vaccine in the elderly. Ann Inter Med 1988;108:653-7.
- 19. Shapiro E.D., Berg A.T., Austrian R., et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med 1991;325:1453-60.
- 20. Buttler J.C., Breiman R.F., Campbell J.F., et al. Pneumococcal polysaccharide vaccine efficacy: An evaluation of current recommendations. JAMA 1993;270:1826-31.
- 21. Sisk J.E., Moskowitz A.J., Whang W., et al. Cost-effectiveness of vaccination against pneumococcal bacteremia among elderly people. JAMA 1997;278:1333.
- 22. Ament A., Baltussen R., Duru G., et al. Cost-effectiveness of pneumococcal vaccination of older people: study in 5 western european countries. Cl Inf Dis 2000;31:444-50.
- 23. Centers for Disease Control. Pneumococcal polysaccharide vaccine usage, United States. MMWR 1984;33:273-86.
Address for correspondence:
Publication Dates
-
Publication in this collection
30 July 2007 -
Date of issue
June 2007
History
-
Reviewed
30 May 2007 -
Received
05 Jan 2007