-
PDF
- Split View
-
Views
-
Cite
Cite
Xenofon Baraliakos, Joachim Listing, Claudia Fritz, Hildrun Haibel, Rieke Alten, Gerd-Rüdiger Burmester, Andreas Krause, Stefan Schewe, Matthias Schneider, Helmut Sörensen, Reinhold Schmidt, Joachim Sieper, Juergen Braun, Persistent clinical efficacy and safety of infliximab in ankylosing spondylitis after 8 years—early clinical response predicts long-term outcome, Rheumatology, Volume 50, Issue 9, September 2011, Pages 1690–1699, https://doi.org/10.1093/rheumatology/ker194
Close - Share Icon Share
Abstract
Objectives. To report for the first time on the efficacy and safety of anti-TNF therapy after 8 years of follow-up in patients with active AS, and analyse possible short-term predictors for long-term clinical outcomes.
Methods. In this open-label extension of a randomized controlled trial, proportions of the initially included 69 patients with active AS were treated with infliximab 5 mg/kg i.v./6 weeks for 8 years. The last report was published after 5 years. All analyses were based on completers.
Results. Overall, 33 (48%) patients completed 8 years. Their mean (s.d.) BASDAI [2.6 (1.9)], BASFI [3.3 (2.6)] and BASMI [2.7 (2.4)] remained low at Year 8. At the end of Year 8, most patients were either in partial remission (n = 8, 24%) or had low disease activity (BASDAI < 3; n = 21, 64%). No new serious adverse events occurred within the past 3 years. Adverse events were the most frequent reason for dropout (56%). There were no differences between completers and dropouts at baseline, but the latter had higher BASFI values at dropout. No baseline parameter was associated with good long-term response to infliximab, but lower BASDAI levels after 12 weeks were predictive of a higher probability of partial remission [odds ratio (OR) 2.9, 95% CI 1.3, 6.3, P = 0.007], low disease activity (OR 1.7, 95% CI 1.2, 2.3, P = 0.005) or remaining on treatment (OR 0.79, 95% CI 0.61, 1.01, P = 0.06) after 8 years.
Conclusion. Almost half of the initially treated patients remained on anti-TNF therapy for 8 years, and almost 90% were in partial remission or had low disease activity. Short-term response (low BASDAI at 3 months) is predictive of outcome after 8 years. Infliximab therapy was safe over 8 years.
Introduction
AS, a frequent chronic inflammatory disease with a high prevalence of ∼0.5% [1–4], represents the prototype of the spondyloarthritides (SpAs). Due to the already partial high burden of disease for young patients [5], direct and indirect costs are substantial [6–8]. Absence from work and work disability are 3-fold increased in AS patients [8–10].
Although many patients respond well to treatment with NSAIDs and physiotherapy [11], >20% of patients still report insufficient pain control and >40% have to change at least one NSAID due to lack of efficacy [12]. For patients with consistently high disease activity (defined as a BASDAI [13] ≥4 U on a 0–10 scale) despite treatment with at least two NSAIDs within 3 months in combination with a positive expert opinion, treatment with anti-TNF blockers [14] has been recommended by the assessment of spondyloarthritis international Society (ASAS) [15]. According to these recommendations, the efficacy of TNF blockers should be evaluated based on BASDAI improvement [>50% from baseline (BL) or improvement of at least 2 U]—in combination with a positive expert opinion.
In the first study assessing the effect of TNF blockade in patients with active AS, infliximab infusions given every 6 weeks at a dosage of 5 mg/kg showed significant clinical improvement of clinical and laboratory disease parameters after 3 months [16]; this effect remained rather stable over time. However, treatment discontinuation after 3 years of continuous therapy led to a clinical relapse in almost all patients, but re-administration of infliximab was safe and efficacious [17]. After 5 years, no loss of efficacy and no new safety issues were observed [18]. MRI examinations of these patients showed a significant decrease in inflammatory spinal lesions in the anti-TNF, but not in the placebo-treated patients [19] after 3 months, while the outcome of the completers after 2 years confirmed this improvement [20].
Nevertheless, despite the proved short-term efficacy of TNF blockers, there is limited knowledge about the long-term outcomes of treatment with infliximab and other biologic agents in patients with not only AS but also in other rheumatological indications, such as RA.
In the present study, we report for the first time on 8-year results of anti-TNF therapy with infliximab in patients with active AS, and analyse possible short-term predictors for long-term clinical outcomes of this treatment. Furthermore, we compare the data of all patients who have completed the 8-year follow-up with those of patients who dropped out early.
Methods
Patients and study protocol
The results of the 12-week, randomized, placebo-controlled phase of the study [16], also of the 1-, 3- and 5-year extensions have been previously reported [17, 18, 21–23]. Briefly, 69 randomly selected patients with severe and active (BASDAI > 4) AS were assigned at BL to receive infliximab 5 mg/kg every 6 weeks with a loading dose at Week 2 or placebo for 12 weeks. Concomitant DMARDs and oral CSs were not allowed during the study. Use of NSAIDs was permitted, and dosages of NSAIDs were allowed to be reduced but not increased during the study. At Week 12, patients initially assigned to placebo switched to infliximab (5 mg/kg/6 weeks with a loading dose at Week 2). All patients continued infliximab treatment for 3 years and were then asked to discontinue infliximab. These data have been described elsewhere [22]. Retreatment was again allowed in cases of clinical flare (BASDAI ≥ 4 and physician’s global assessment ≥ 4) according to the ASAS recommendations [24] with infliximab at the same dosage and with the same intervals. Most patients relapsed within 18–24 weeks [18], some patients needed 1 year until relapse and retreatment. At the end of Year 5 of the study, all but one patient had been retreated with infliximab for at least 1 year [18].
The study medication was manufactured by Centocor Inc. (Malvern, USA), and it was packaged and labelled by Essex Pharma (Munich, Germany). The ethics committees from the participating centres (universities of Berlin, Hannover, Düsseldorf and Munich) approved the original study as well as the current extensions. All patients participating in this extension gave written informed consent.
Assessments
The disease status was assessed by using validated parameters for disease activity (BASDAI), metrology (BASMI [25]) and function (BASFI [26]). A status of low disease activity was defined as BASDAI < 3 U at the assessed time points, as reported previously [18]. All parameters were measured using a numeric rating scale (NRS) ranging from 0 to 10. Assessment of peripheral arthritis was performed by counting the number of swollen joints (total of 64 joints) [16]. Other reasons for joint pain, tenderness or swelling had to be excluded clinically and/or by imaging if necessary. Laboratory parameters for inflammation were measured in all patients with CRP and ESR.
Partial remission was defined, according to ASAS, as a score ≤2 (on a scale of 0–10) in each of the four ASAS domains [27]: patient’s global assessment, pain, function (represented by the BASFI score) and morning stiffness (represented by the mean of the two morning stiffness scales of the BASDAI). Other assessments for treatment response were a 50% improvement in the BASDAI, a 40% improvement in the ASAS improvement criteria and calculation of ASAS 5/6 criteria [28]. To meet the latter criteria, a 20% improvement in any five of the following six domains is required: the four domains used for partial remission, and in addition spinal mobility (as assessed by the BASMI) and CRP as acute-phase reactant.
We investigated the outcome in patients who completed the 8 years [follow-up 2 (FU2)] of follow-up (n = 33), compared them with those patients who did not complete (n = 35), and investigated the association between short-term response (BASDAI after 3 months of treatment with infliximab) and the outcome after 8 years in the complete intention-to-treat population (n = 69). In the completer analysis, the state of disease activity and function as well as clinical response (low disease activity defined as BASDAI < 3, ASAS 20%, ASAS 40%, ASAS 5/6, BASDAI 50%, all compared with BL) and ASAS partial remission at the end of the eighth study year were investigated.
All clinical outcomes were compared with the status of the patients at BL, after the first 3 months of the study (end of the placebo-controlled phase) and also Year 5 of the study. The latter time point was chosen since it included all patients with at least 1 year of retreatment after infliximab discontinuation.
Statistical analysis
The t-test and Fisher’s exact test were used to compare group effects and as appropriate for small sample sizes the Blyth–Still–Casella method was applied to calculate 95% CIs of response rates. The last valid BASDAI (BASFI, etc.) value of dropouts was compared with those of the completers at the corresponding time point by the application of a t-test type dropout test as proposed by Listing et al. [29]. Logistic regression and categorical regression analysis were used to investigate the predictive value of the BASDAI after 12 weeks of treatment with infliximab for the outcomes after 8 years of follow-up in the intention-to-treat population.
Results
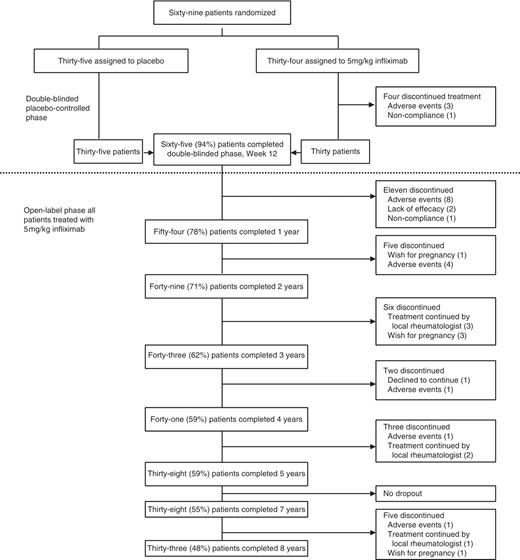
From the initially 69 patients included in the study, 65 (94%) patients completed 3 months (30 on infliximab and 35 on placebo), while 38 (55%) finished Year 5, and 33 (48%) Year 8 of the study—51% of the completers of Month 3 and 87% of those who completed Year 5 (Fig. 1). A detailed analysis of the reasons for discontinuation is given below. The BL characteristics of all completers are shown in Table 1, together with those of patients who discontinued treatment before reaching Year 8 of the study.
Summary of patient disposition through the entire study period of 8 years.
Baseline characteristics of patients who completed the eighth year of the study (completers) and of those who dropped out before end of Year 8 (dropouts)
| Characteristic . | Completers (n = 33) . | Dropouts (n = 36) . | P-value . |
|---|---|---|---|
| Gender: male, % | 75.8 | 55.6 | 0.128a |
| HLA-B27 pos., % | 96.9 | 83.3 | 0.110a |
| Age, years | 37.8 (8.0) | 41.3 (8.9) | 0.603b |
| BASDAI | 6.4 (1.4) | 6.4 (1.2) | 0.119b |
| BASFI | 5.4 (1.8) | 5.2 (2.2) | 0.256b |
| BASMI | 3.7 (1.9) | 3.6 (2.2) | 0.703b |
| CRP (mg/l) | 29.0 (20.7) | 25.8 (29.8) | 0.171c |
| ESR (mm/1 h) | 30.6 (20.9) | 33.1 (23.7) | 0.843c |
| Characteristic . | Completers (n = 33) . | Dropouts (n = 36) . | P-value . |
|---|---|---|---|
| Gender: male, % | 75.8 | 55.6 | 0.128a |
| HLA-B27 pos., % | 96.9 | 83.3 | 0.110a |
| Age, years | 37.8 (8.0) | 41.3 (8.9) | 0.603b |
| BASDAI | 6.4 (1.4) | 6.4 (1.2) | 0.119b |
| BASFI | 5.4 (1.8) | 5.2 (2.2) | 0.256b |
| BASMI | 3.7 (1.9) | 3.6 (2.2) | 0.703b |
| CRP (mg/l) | 29.0 (20.7) | 25.8 (29.8) | 0.171c |
| ESR (mm/1 h) | 30.6 (20.9) | 33.1 (23.7) | 0.843c |
There were no significant differences in any of the characteristics between the two groups. Values are given in percentage (%) or in units [mean (s.d.)]
aFisher’s test. bt-test. cMann–Whitney U-test.
Baseline characteristics of patients who completed the eighth year of the study (completers) and of those who dropped out before end of Year 8 (dropouts)
| Characteristic . | Completers (n = 33) . | Dropouts (n = 36) . | P-value . |
|---|---|---|---|
| Gender: male, % | 75.8 | 55.6 | 0.128a |
| HLA-B27 pos., % | 96.9 | 83.3 | 0.110a |
| Age, years | 37.8 (8.0) | 41.3 (8.9) | 0.603b |
| BASDAI | 6.4 (1.4) | 6.4 (1.2) | 0.119b |
| BASFI | 5.4 (1.8) | 5.2 (2.2) | 0.256b |
| BASMI | 3.7 (1.9) | 3.6 (2.2) | 0.703b |
| CRP (mg/l) | 29.0 (20.7) | 25.8 (29.8) | 0.171c |
| ESR (mm/1 h) | 30.6 (20.9) | 33.1 (23.7) | 0.843c |
| Characteristic . | Completers (n = 33) . | Dropouts (n = 36) . | P-value . |
|---|---|---|---|
| Gender: male, % | 75.8 | 55.6 | 0.128a |
| HLA-B27 pos., % | 96.9 | 83.3 | 0.110a |
| Age, years | 37.8 (8.0) | 41.3 (8.9) | 0.603b |
| BASDAI | 6.4 (1.4) | 6.4 (1.2) | 0.119b |
| BASFI | 5.4 (1.8) | 5.2 (2.2) | 0.256b |
| BASMI | 3.7 (1.9) | 3.6 (2.2) | 0.703b |
| CRP (mg/l) | 29.0 (20.7) | 25.8 (29.8) | 0.171c |
| ESR (mm/1 h) | 30.6 (20.9) | 33.1 (23.7) | 0.843c |
There were no significant differences in any of the characteristics between the two groups. Values are given in percentage (%) or in units [mean (s.d.)]
aFisher’s test. bt-test. cMann–Whitney U-test.
Patient states and response to treatment for completers after 8 years
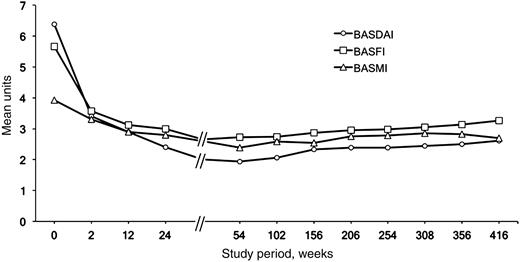
Patients who completed the study showed a status of low disease activity and good functional capacity (Fig. 2). After 8 years, the mean (s.d.) values for BASDAI, BASFI and BASMI were 2.6 (1.9), 3.3 (2.6) and 2.7 (2.4), respectively; the mean (s.d.) CRP level was 2.8 (3.8) mg/l. The comparison of these values with those observed after 12 weeks of treatment showed almost no difference for BASMI and BASDAI, but a slight increase in mean BASFI (0.12 U, 95% CI −0.42, +0.66) values (Fig. 2). This difference was even statistically significant when the mean BASFI levels during the first and the last year of the study were compared (mean difference 0.35 U, 95% CI 0.07, 0.62, P < 0.05).
Course of clinical parameters during the 8 years of the study, including the time of infliximab discontinuation (Year 3 of the study) and retreatment.
To further investigate this in detail, we stratified all completers into two groups: Group A included patients with a BASFI increase of ≥1 U (n = 8), while Group B included patients with either a BASFI increase of <1 U or with BASFI improvement (n = 25) during the time period Week 12 to Year 8. The comparison of the two groups revealed significant differences in the BL radiographic damage of the patients, showing that patients in Group A also had an increased number of structural changes at study entry, as compared with patients in Group B (Table 2).
Comparison of the radiographic damage at BL for the two groups with different BASFI course during 8 years of the study
| Radiographic damage . | Group A (n = 8) . | Group B (n = 22) . | P-value . |
|---|---|---|---|
| mSASSS units at BL—Rater 1, mean (s.d.) | 26.5 (17.2) | 11.3 (15.1) | 0.043 |
| mSASSS units at BL—Rater 2, mean (s.d.) | 30.3 (20.3) | 9.7 (15.1) | 0.016 |
| Radiographic damage . | Group A (n = 8) . | Group B (n = 22) . | P-value . |
|---|---|---|---|
| mSASSS units at BL—Rater 1, mean (s.d.) | 26.5 (17.2) | 11.3 (15.1) | 0.043 |
| mSASSS units at BL—Rater 2, mean (s.d.) | 30.3 (20.3) | 9.7 (15.1) | 0.016 |
Assessment of radiographic progression was done by using the modified Stokes AS spinal score (mSASSS). Two readers scored independently the X-ray images of the patients in a blinded fashion. Group A: patients with worsening of BASFI ≥1 U, Group B: patients with worsening of BASFI <1 U during the 8 years of follow-up. Overall, patients with BASFI worsening ≥1 U had significantly higher BL mSASSS (more structural damage) as compared with patients with very little BASFI worsening (<1 U) during Years 1 and 8 of the study.
Comparison of the radiographic damage at BL for the two groups with different BASFI course during 8 years of the study
| Radiographic damage . | Group A (n = 8) . | Group B (n = 22) . | P-value . |
|---|---|---|---|
| mSASSS units at BL—Rater 1, mean (s.d.) | 26.5 (17.2) | 11.3 (15.1) | 0.043 |
| mSASSS units at BL—Rater 2, mean (s.d.) | 30.3 (20.3) | 9.7 (15.1) | 0.016 |
| Radiographic damage . | Group A (n = 8) . | Group B (n = 22) . | P-value . |
|---|---|---|---|
| mSASSS units at BL—Rater 1, mean (s.d.) | 26.5 (17.2) | 11.3 (15.1) | 0.043 |
| mSASSS units at BL—Rater 2, mean (s.d.) | 30.3 (20.3) | 9.7 (15.1) | 0.016 |
Assessment of radiographic progression was done by using the modified Stokes AS spinal score (mSASSS). Two readers scored independently the X-ray images of the patients in a blinded fashion. Group A: patients with worsening of BASFI ≥1 U, Group B: patients with worsening of BASFI <1 U during the 8 years of follow-up. Overall, patients with BASFI worsening ≥1 U had significantly higher BL mSASSS (more structural damage) as compared with patients with very little BASFI worsening (<1 U) during Years 1 and 8 of the study.
A state of low disease activity (BASDAI < 3) was reached by 21 (63.6%) out of 33 completers after 8 years—very similar to the results after 3 months [20 (60.6%) out of 33 patients] and equal to the results after 5 years. Interestingly, 15 (71.4%) of those 21 patients had BASDAI values <3 at both Month 3 and Year 5 of the study.
Response to treatment after 8 years of infliximab therapy
As compared with BL, an ASAS 20% response was achieved by 28 (84.8%) out of 33 patients at Year 8 of the study, which is the same as after 3 months and similar to the 27 (81.8%) out of 33 patients after 5 years, while a BASDAI 50% response was seen in 19 (57.56%) out of 33 patients after 3 months, in 23 (69.7%) out of 33 patients after 5 years and in 21 (63.6%) out of 33 patients after 8 years. The response rates for all assessments at important time points of the study are shown in Table 3.
Treatment outcome at the most important time points of the study for all 33 patients who completed the study period of 8 years
| . | 12 weeks . | 1 year . | 3 years . | 5 years . | 8 years . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . |
| ASAS 20 | 28 (84.8) | 69.2, 93.8 | 30 (90.9) | 77.1, 97.5 | 31 (93.9) | 80.8, 98.9 | 27 (81.8) | 66.2, 91.8 | 28 (84.8) | 69.2, 93.8 |
| ASAS 40 | 24 (72.7) | 54.9, 85.3 | 28 (84.8) | 69.2, 93.8 | 24 (72.7) | 54.9, 85.3 | 22 (66.7) | 48.2, 80.8 | 21 (63.6) | 45.1, 79.1 |
| ASAS 5/6 | 24 (72.7) | 54.9, 85.3 | 28 (84.8) | 69.2, 93.8 | 25 (75.8) | 57.9, 88.3 | 23 (69.7) | 51.8, 84.4 | 24 (72.7) | 54.9, 85.3 |
| BASDAI 50 | 19 (57.6) | 40.1, 73.2 | 26 (78.8) | 62.2, 90.8 | 22 (66.7) | 48.2, 80.8 | 23 (69.7) | 51.8, 84.4 | 21 (63.6) | 45.1, 79.1 |
| ASAS partial remission | 6 (18.2) | 8.2, 33.8 | 11 (33.3) | 19.2, 51.8 | 13 (39.4) | 22.9, 57.9 | 12 (36.4) | 20.9, 54.9 | 8 (24.2) | 11.7, 42.1 |
| . | 12 weeks . | 1 year . | 3 years . | 5 years . | 8 years . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . |
| ASAS 20 | 28 (84.8) | 69.2, 93.8 | 30 (90.9) | 77.1, 97.5 | 31 (93.9) | 80.8, 98.9 | 27 (81.8) | 66.2, 91.8 | 28 (84.8) | 69.2, 93.8 |
| ASAS 40 | 24 (72.7) | 54.9, 85.3 | 28 (84.8) | 69.2, 93.8 | 24 (72.7) | 54.9, 85.3 | 22 (66.7) | 48.2, 80.8 | 21 (63.6) | 45.1, 79.1 |
| ASAS 5/6 | 24 (72.7) | 54.9, 85.3 | 28 (84.8) | 69.2, 93.8 | 25 (75.8) | 57.9, 88.3 | 23 (69.7) | 51.8, 84.4 | 24 (72.7) | 54.9, 85.3 |
| BASDAI 50 | 19 (57.6) | 40.1, 73.2 | 26 (78.8) | 62.2, 90.8 | 22 (66.7) | 48.2, 80.8 | 23 (69.7) | 51.8, 84.4 | 21 (63.6) | 45.1, 79.1 |
| ASAS partial remission | 6 (18.2) | 8.2, 33.8 | 11 (33.3) | 19.2, 51.8 | 13 (39.4) | 22.9, 57.9 | 12 (36.4) | 20.9, 54.9 | 8 (24.2) | 11.7, 42.1 |
12 weeks: time period for evaluation of treatment efficacy of biologics, according to ASAS (for patients enrolled into the placebo group, the outcome after 12 weeks of treatment with infliximab was used for analysis); 1 year: first long-term time point for efficacy of treatment; 3 years: time point before discontinuation of treatment in this study; 5 years: long-term time point for efficacy evaluation after retreatment; 8 years: time point of treatment evaluation in this study, first report on 8-year data of treatment with a TNF blocker in patients with AS. ASAS 20, 40, 5/6; partial remission: percentage of patients achieving ASAS partial remission. BASDAI 50: percentage of patients showing improvement in BASDAI of ≥ 50% as compared with the BL BASDAI level.
Treatment outcome at the most important time points of the study for all 33 patients who completed the study period of 8 years
| . | 12 weeks . | 1 year . | 3 years . | 5 years . | 8 years . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . |
| ASAS 20 | 28 (84.8) | 69.2, 93.8 | 30 (90.9) | 77.1, 97.5 | 31 (93.9) | 80.8, 98.9 | 27 (81.8) | 66.2, 91.8 | 28 (84.8) | 69.2, 93.8 |
| ASAS 40 | 24 (72.7) | 54.9, 85.3 | 28 (84.8) | 69.2, 93.8 | 24 (72.7) | 54.9, 85.3 | 22 (66.7) | 48.2, 80.8 | 21 (63.6) | 45.1, 79.1 |
| ASAS 5/6 | 24 (72.7) | 54.9, 85.3 | 28 (84.8) | 69.2, 93.8 | 25 (75.8) | 57.9, 88.3 | 23 (69.7) | 51.8, 84.4 | 24 (72.7) | 54.9, 85.3 |
| BASDAI 50 | 19 (57.6) | 40.1, 73.2 | 26 (78.8) | 62.2, 90.8 | 22 (66.7) | 48.2, 80.8 | 23 (69.7) | 51.8, 84.4 | 21 (63.6) | 45.1, 79.1 |
| ASAS partial remission | 6 (18.2) | 8.2, 33.8 | 11 (33.3) | 19.2, 51.8 | 13 (39.4) | 22.9, 57.9 | 12 (36.4) | 20.9, 54.9 | 8 (24.2) | 11.7, 42.1 |
| . | 12 weeks . | 1 year . | 3 years . | 5 years . | 8 years . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . | n (%) . | 95% CI . |
| ASAS 20 | 28 (84.8) | 69.2, 93.8 | 30 (90.9) | 77.1, 97.5 | 31 (93.9) | 80.8, 98.9 | 27 (81.8) | 66.2, 91.8 | 28 (84.8) | 69.2, 93.8 |
| ASAS 40 | 24 (72.7) | 54.9, 85.3 | 28 (84.8) | 69.2, 93.8 | 24 (72.7) | 54.9, 85.3 | 22 (66.7) | 48.2, 80.8 | 21 (63.6) | 45.1, 79.1 |
| ASAS 5/6 | 24 (72.7) | 54.9, 85.3 | 28 (84.8) | 69.2, 93.8 | 25 (75.8) | 57.9, 88.3 | 23 (69.7) | 51.8, 84.4 | 24 (72.7) | 54.9, 85.3 |
| BASDAI 50 | 19 (57.6) | 40.1, 73.2 | 26 (78.8) | 62.2, 90.8 | 22 (66.7) | 48.2, 80.8 | 23 (69.7) | 51.8, 84.4 | 21 (63.6) | 45.1, 79.1 |
| ASAS partial remission | 6 (18.2) | 8.2, 33.8 | 11 (33.3) | 19.2, 51.8 | 13 (39.4) | 22.9, 57.9 | 12 (36.4) | 20.9, 54.9 | 8 (24.2) | 11.7, 42.1 |
12 weeks: time period for evaluation of treatment efficacy of biologics, according to ASAS (for patients enrolled into the placebo group, the outcome after 12 weeks of treatment with infliximab was used for analysis); 1 year: first long-term time point for efficacy of treatment; 3 years: time point before discontinuation of treatment in this study; 5 years: long-term time point for efficacy evaluation after retreatment; 8 years: time point of treatment evaluation in this study, first report on 8-year data of treatment with a TNF blocker in patients with AS. ASAS 20, 40, 5/6; partial remission: percentage of patients achieving ASAS partial remission. BASDAI 50: percentage of patients showing improvement in BASDAI of ≥ 50% as compared with the BL BASDAI level.
Analysis of safety and the reasons for treatment discontinuation over 8 years
There were no differences in BL status between those patients who completed the eighth year of the study and those who had to drop out of the study before Year 8 (Table. 1). Overall, 36 patients dropped out of the study due to adverse events (n = 20), treatment prescribed by the local rheumatologist (n = 6), wish for pregnancy (n = 5), non-compliance (n = 2) and lack of response (n = 3, all of the latter within the first year of the study). The most frequent adverse event that led to a dropout from the study was an increase in transaminases and in ANAs (n = 8 patients), followed by infusion reactions (n = 3 patients) and loss of efficacy (n = 3), while there was one patient who discontinued for each of the following diagnoses: tuberculosis (during the first 3 months of the study), positive testing for tuberculosis and refusing prophylaxis treatment, development of allergic granulomatosis of the lung, pancreatitis and worsening of general well-being after infliximab infusions.
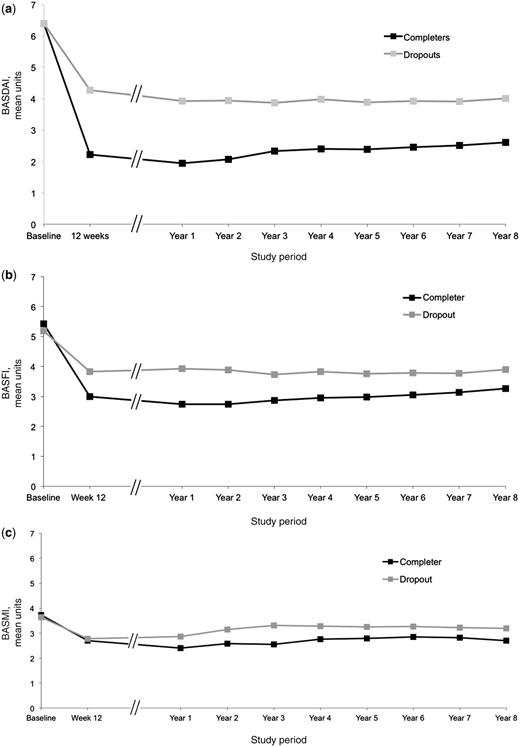
Overall, patients who had discontinued the study within 8 years had significantly higher mean values for disease activity and function at the individual time points or discontinuation as compared with patients who completed Year 8. The mean (s.d.) difference within those groups was 1.6 (0.3) (P < 0.001) for BASDAI and 0.8 (0.3) (P = 0.01) for BASFI, while the mean (s.d.) difference in BASMI was not significant [0.5 (0.3) U between groups] (Fig. 3).
Course of clinical parameters during the 8 years of the study for BASDAI (a), BASFI (b) and BASMI (c), comparing the mean values of all completers (Completers, completer analysis, n = 33) and patients who dropped out of the study before reaching Year 8 (Dropouts, n = 36, last operation carried forward analysis). Overall, patients who had discontinued the study had significantly higher mean values for disease activity and function as compared with patients who completed Year 8.
Prediction of response
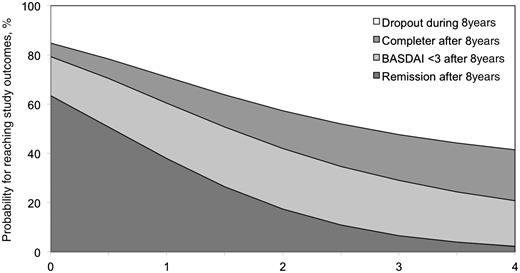
No significant association between any of the BL indices was found for prediction of long-term response to infliximab treatment. However, short-term improvement was associated with higher probability of remaining on treatment and achieving a status of low disease activity (BASDAI < 3) or being in a state of partial remission after 8 years (Fig. 4). Using univariate logistic regression analysis, a strong tendency or a significant association was seen between the BASDAI status after 12 weeks of treatment and the probability of: A significant association between BASDAI status after 12 weeks and 8 years of infliximab treatment was also found by categorical regression analysis for all three possible outcomes (P = 0.0246). These results correspond to the probabilities for the different outcomes in dependency from BASDAI status as shown in Fig. 4.
remaining on treatment and achieving partial remission [odds ratio (OR) per unit increase in BASDAI]: 0.34, 95% CI 0.16, 0.75, P = 0.007];
reaching a state of low disease activity (OR 0.60, 95% CI 0.43, 0.86, P = 0.005); or
remaining on treatment (OR 0.79, 95% CI 0.61, 1.01, P = 0.06).
Prediction for reaching different study outcomes after 8 years, depending on the BASDAI status after 3 months of infliximab treatment. In this figure, different treatment outcomes were defined as: state of partial remission (remission), state of low disease activity (BASDAI <3), ongoing treatment medication in any status (Completer) or dropout of the study before Year 8 (Dropout). As an example, a patient with a BASDAI of 1 U after 3 months of infliximab treatment had an estimated probability of 63% of being in partial remission and an estimated probability of 79% of achieving low disease activity (BASDAI < 3) after 8 years of treatment, while the probability of dropping out of the study was 17%. On the other hand, a patient with a BASDAI of 4 after 3 months of infliximab treatment had an estimated probability of only 2.3% of being in partial remission and an estimated probability of only 20.8% of achieving low disease activity (BASDAI < 3), while the dropout probability after 8 years of infliximab treatment was 68%.
Peripheral, organ and other symptoms
Enthesitis was observed in 16 (48.5%) out of 33 patients at BL as compared with 6 (18.2%) patients after 8 years. The mean (s.d.) amount of enthesitic sites decreased from 2.1 (3.5) at BL to 0.7 (2.1) at Year 8 (P = 0.001). Similarly, peripheral arthritis was seen in 11 (33.3%) out of 33 patients at BL as compared with 4 (12.1%) out of 33 patients after 8 years, with a mean (s.d.) number of arthritic joints of 2.7 (6.5) at BL and 0.6 (1.9) at Year 8 (P = 0.001). Both the physical and the mental component of Short-Form 36 questionnaire (SF-36) increased from 28.9 (7.1) and 43.2 (11.8) at BL to 37.8 (10.8) and 50.3 (9.5) at Year 8, respectively.
A history of anterior uveitis (AU) was reported by 12 (36%) out of 33 patients at BL. During the 8 years of the study, six flares of AU in 4 of the 33 completers were observed, while there was no new onset of AU. There were two patients with two AU flares and another two with one flare during the study period. In more detail, four of those flares occurred during the first 5 years of the study and two flares occurred between Years 5 and 8. All flares resolved without complications.
Discussion
This study is the first to report on 8 years of anti-TNF treatment in patients with AS. The study shows that therapy of AS patients with the TNF blocker infliximab is efficacious and safe over 8 years. Clinical improvement of outcomes related to disease activity, function and mobility was largely maintained over 8 years, and this even included a period of discontinuation and re-administration of treatment after 3 years; these data have been reported elsewhere [17]. In the meantime, all patients in this study were retreated after discontinuation, the last one after 5 years. These results suggest on the one hand that continuous treatment seems to be necessary in the vast majority of patients with active AS but, on the other hand, it seems also possible to discontinue and restart therapy after some years without loss of treatment response in the long term. Since, in contrast to RA, DMARDs or glucocorticoids are not efficacious in AS [14], anti-TNF treatment with infliximab or other TNF blockers [30–32] can be considered a major step forward in the management of AS [14].
The main outcome parameter in this 8-year study was patient-derived disease-related clinical parameters, such as disease activity (measured by the BASDAI), function (measured by the BASFI) and metrology (measured by the BASMI). All these three had already shown significant improvement at Week 2 of the study [16], continued to improve during the first 6 months and remained at similar levels over the entire study period, indicating no loss of efficacy of infliximab in this patient group over 8 years. At this time point, the vast majority of the patients (almost 90%) were either in partial remission or in a state of low disease activity with mean (s.d.) BASDAI levels of 2.6 (1.9) (below the proposed cut-off for definition of low disease activity with BASDAI <3). Expectedly, the sustained efficacy of infliximab was also observed with other response measures such as BASDAI 50, ASAS 20, ASAS 40 and ASAS 5/6.
However, at the end of Year 8 of the study, we observed a slight increase in BASFI levels in comparison with previous years. This slight decline in function is also a possible explanation for the decrease in the proportion of patients in partial remission that was observed in the last year of this study. Interestingly, the patients with BASFI increases >1 U after 8 years had more radiographic damage at BL. The significance of radiographic damage at BL in AS has been recently discussed [33]. The data presented may suggest that the reason for the mean deterioration of function is rather the structural damage that has occurred over 8 years than the loss of anti-inflammatory efficacy. However, this hypothesis clearly needs further study. If this holds true, it would clearly support recent data that have not seen a major effect of anti-TNF therapy on radiographic progression in AS [34–36]. It may well take several years, until new bone formation becomes functionally relevant in AS.
Importantly, the improvement in mobility as assessed by objective clinical parameters (BASMI) was largely maintained over time. The significance of this finding over a shorter period of time, also in relation to structural parameters, has recently been discussed in detail [37]. It is important to stress in this regard that the natural course of patients with AS who are not treated with TNF blockers usually shows continuous worsening of function and mobility over time [38, 39].
We were not able to adjust the treatment doses of infliximab to those patients who either showed no response at 12 weeks or showed a worsening of any of the disease assessments over time, since the study protocol did not allow for such change. Data from the Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy (ASSERT study) [40] suggest some improvement in the percentage of ASAS responders 12 weeks after an increase in infliximab to 7.5 mg/kg/6 weeks for those patients who had no ASAS response with the initial dose of 5 mg/kg/weeks in the first 12 weeks of treatment in that study. Nevertheless, since this change was not part of the protocol in the present study, we cannot make any conclusions about the effect of dose adjustment on the long-term outcome of our patients.
Another important result of this study is that the BASDAI response or more precise a low BASDAI value at Month 3 was identified as the most predictive measure for a favourable long-term response (partial remission and low disease activity) after 8 years of treatment. To our knowledge, this study is the first to show that short-term efficacy (as assessed by BASDAI) of anti-TNF treatment is predictive of clinically relevant long-term outcomes. This finding strongly supports the guidelines published by ASAS for monitoring and continuation of treatment after initiation of TNF blockers in AS [15, 24], where it is recommended that the monitoring of patients under anti-TNF therapy should include regular BASDAI assessments and a negative decision on continuation of treatment in non-responders after 6–12 weeks. Thus, non-responders should not receive the same anti-TNF agent for >3 months—especially since it was shown that switching is possible and has relatively high response rates [41], although less so in the primary non-responders. However, possible alternative approaches targeting IL-6 and IL-17 are currently under investigation.
There was no correlation of the BL clinical status of the patients and their treatment response or maintenance of treatment after 8 years. This is in contrast to some previous data [42], where shorter disease duration [43], younger age, lower BASFI, raised CRP and higher BASDAI were identified as major predictors of a major (BASDAI 50) clinical response to TNF blockers in patients with active AS. We explain this missing correlation by the relatively low number of completers after 8 years; in earlier studies more patients had been included [40, 44, 45].
It remains to be seen in future long-term analyses whether the recently published new ASDAS [46] as well as its recently presented cut-offs for different stages of disease activity [47] will show similar results. A clear advantage of the ASDAS is its data-driven basis. We have shown recently [48] that both the BASDAI and the ASDAS have good agreement on status and on change scores for disease activity during long-term treatment with anti-TNF. Since the current consensus statement is still based on the BASDAI as the major outcome parameter, which is currently internationally used in all countries, we have decided to present the data based on this tool in the present study.
With respect to the long-term safety and the reasons for treatment discontinuation during these 8 years of the study, the dropout rate in this long-term follow-up reached 52% (n = 36) of the 69 patients who were initially included. Interestingly, up to Year 7, the number of patients who remained in the study did not differ from those who finished Year 5 (n = 38), while five patients dropped out during the last study year. One of those five patients was in partial remission at the time point of dropout, while the reasons for discontinuation were mainly infusion-related reactions. However, a substantial proportion of the patients who discontinued treatment did that based on reasons not related to complications with the study drug, since they were reimbursed by their rheumatologist and wish for pregnancy (both together leading to a dropout rate of 31% during the 8 years of the study). Finally, only some patients (n = 5) discontinued due to lack of response or non-compliance. These results are positive and in favour not only of the treatment with infliximab, but also anti-TNF treatment in general.
In summary, there is good evidence for largely sustained efficacy and a good safety profile of anti-TNF therapy in AS. As recommended by ASAS, the efficacy of treatment with TNF blockers should be evaluated after 3 months. The treatment outcome after this time is a reliable predictor of long-term outcome after at least 8 years.
Acknowledgements
We would like to thank the medical staff and study nurses of all participating sites for their tireless work and cooperation in this study and especially B. Buss, I.-H. Song, C. Herz, K. Mattat, A Müller, J. Richter, E. Richter, B. Andermann, S. Dyanchenko, C. Pohl and C. Strasser.
Disclosure statement: X.B. has received honoraria and grants/research support from MSD and Essex Pharma. H.H. has received speakers’ honoraria of less than 10 000 Euro from MSD, Pfizer and Abbott. A.K. has received honoraria and is a consultant for MSD, Essex, Pfizer, Wyeth and Abbott. G.-R.B. is a consultant for, received honoraria from and is a member of a speakers’ bureau for Essex Pharma and MSD. R.A. has received research grants and honoraria from Abbott, BMS, Centocore, MSD, Novartis, Pfizer, Roche and UCB. R.S. has received grants/research support as well as honoraria as a consultant from Chugai/Roche. J.S. has received honoraria and research support from Merck and Centocor. J.B. is a consultant for, has received honoraria, grant/research support from and is a member of a speakers’ bureau for Merck and Centocor. All other authors have declared no conflicts of interest.




Comments