-
PDF
- Split View
-
Views
-
Cite
Cite
Karin Thursky, Joseph Torresi, Primary Muscle Hydatidosis of the Thigh: Management of a Complicated Case with Combination Adjunctive Albendazole and Praziquantel Chemotherapy, Clinical Infectious Diseases, Volume 32, Issue 3, 1 February 2001, Pages e65–e68, https://doi.org/10.1086/318521
Close - Share Icon Share
Abstract
A patient had primary muscle hydatidosis of the thigh that was not detected radiologically or by fine-needle aspiration before surgery. The risk of dissemination during the initial exploratory procedure was high. Treatment consisted of formal muscle resection and combination therapy with albendazole and praziquantel. Clinical features of muscle hydatidosis and the role of adjunctive chemotherapy are reviewed.
Primary skeletal muscle hydatidosis is a rare manifestation of cystic hydatid disease [1]. Skeletal muscle cysts are commonly misdiagnosed as either a malignancy or pyogenic infection because radiological appearances are not specific and hydatid serology often yields negative results. Management of muscular hydatidosis is largely surgical. However, there is an increasing role for adjuvant chemotherapy with albendazole. We report a case of muscular hydatid disease complicated by intraoperative spillage of cysts treated postoperatively with a combination of albendazole and praziquantel.
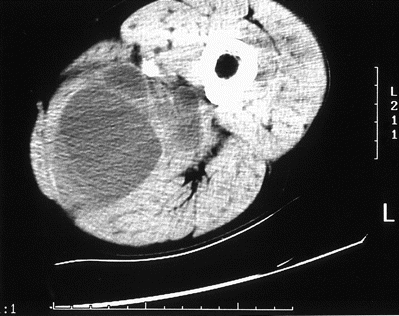
Case report. A 63-year-old Greek man was admitted to the general surgical unit complaining of a painful swelling of the left thigh. Before immigrating to Australia at the age of 20, he had worked in agriculture in Greece. After coming to Melbourne, he worked in the laundering industry and had no further contact with domestic livestock. The swelling developed over 3 days and was associated with fever, rigors, and pruritus of the thigh. He had recently returned from a holiday in Queensland, but he denied a history of bites or trauma or previous symptoms related to the thigh. He was afebrile on admission, and examination revealed a tender swollen medial left thigh with erythema and excoriation of the overlying skin. Investigations demonstrated a normal total WBC count of 5.0 × 109 cells/L, eosinophilia of 0.66 × 109 cells/L, and an erythrocyte sedimentation rate of 80 mm/h. A provisional diagnosis of pyomyositis was made, and treatment with iv flucloxacillin was commenced. Contrast-enhanced CT on the following day demonstrated a loculated noncalcified cystic mass in the posteromedial muscle compartment of the left thigh (figure 1). On day 3, he became febrile with a temperature of 38.8°C. Ultrasound-guided aspiration was done, with drainage of 100 mL of purulent material, and a drainage catheter was left in situ. Gram's staining showed pus cells but no organisms, and cultures of the fluid failed to yield any pathogens. On day 6, he was referred to the infectious diseases unit because of persistent fever and marked swelling and induration of the adductor compartment.
CT of thigh demonstrating loculated fluid in posteromedial muscle compartment.
Formal drainage of the supposed abscess was done on the following day. A multiloculated cavity containing multiple cysts consistent with hydatid disease was found. Histological examination of operative specimens confirmed Echinococcus granulosus. Treatment with albendazole at 400 mg b.i.d. was immediately commenced postoperatively. No additional cysts were found on CT of the abdomen and pelvis.
Because of the significant risk of dissemination of daughter cysts and protoscoleces from the initial surgical intervention, formal exploration and excision of the cyst remnants were planned after 1 week of albendazole therapy. The adductor longus, brevis, and magnus muscles were resected in toto, and the sartorius and gracilis muscles were preserved (figure 2). At surgery, the cyst was seen to extend up into the femoral canal, making its complete excision without accidental spillage of daughter cysts not possible. The wound was lavaged with hypertonic saline, and hydrogen peroxide was instilled for 5 min.
Photograph of formal cyst/muscle resection showing empty cyst (left) and edematous gracilis muscle (right).
The immediate postoperative course was complicated by anemia associated with hypotension, requiring transfusion of 2 units of red blood cells. The hypotension occurred several hours after surgery and was not considered to be the result of anaphylaxis from spillage of cyst contents. Although his fever, pain, and induration resolved initially, he developed a wound infection after 2 weeks, requiring further surgical débridement and antibiotic therapy with vancomycin in addition to timentin. Four weeks after the initial cyst excision, he required resection of devascularized semimembranosis muscle. At surgery, multiple fluid-filled cysts were seen adherent to the femoral vessels and extending up into the femoral canal. Biopsies were not done, but the appearance was thought to be consistent with disseminated hydatid cysts. Chemotherapy was changed to therapy with continuous albendazole and intermittent praziquantel at 40 mg/kg that alternated between 1 week on and 1 week off. After completing 3 cycles of praziquantel with 3 months of albendazole treatment, the patient made an uneventful recovery. The wound was allowed to heal by secondary intention and revised 10 months later with a skin graft. After 18 months of follow-up, he exhibited no local or systemic signs of recurrence, and repeat CT of the thigh showed no evidence of recurrent hydatid disease. The patient now remains under continuing follow-up for potential recurrence at a later date.
Hydatid antibody testing by means of immunoelectrophoresis yielded negative results on all occasions. On gel immunoelectrophoresis, he had a single band at presentation, which increased to 2 bands after surgery and returned to a single band by 3 months; however, arc 5 was absent throughout.
Discussion and literature review. This case of primary muscular hydatidosis illustrates the significant morbidity associated with complicated hydatid disease. The diagnosis was not detected radiologically or after fine-needle aspiration before surgery. As a consequence, the risk of dissemination of daughter cysts during the initial exploratory procedure was high. We believe that this is the first report of muscular hydatid disease treated with the adjunctive combination of albendazole and praziquantel together with surgical intervention. The treatment was well tolerated and may provide an important addition to albendazole monotherapy in the perioperative management of large extrahepatic hydatid cysts that are not amenable to complete surgical excision.
In Australia, surveys in regions with a high incidence of human hydatidosis reveal a high prevalence of infection in definitive hosts—domestic and wild dogs (including dingoes)—and in intermediate hosts—sheep, macropods (kangaroos and wallabies), and feral pigs [2]. Human hydatidosis in Australia is seen in 2 main patient groups: rural patients, who are mostly Australian born, and urban patients, who are immigrants. In a review of 152 urban cases diagnosed in New South Wales from 1987 through 1992, the majority were in Lebanese and Balkan immigrants (particularly those from Greece and the former Republic of Yugoslavia) [3]. From 1992 through 1996 in Victoria, there were 68 notifications of hydatid disease [4]. The incidence of reported infections in humans for 1992 was 0.23 per 100,000 for the whole of Australia [2]. Our patient had no known risk exposures after immigrating to Australia, and given the low incidence of hydatid disease in Australia, it was likely that the infection was acquired in Greece before the patient immigrated.
Primary skeletal muscle infection with E. granulosus accounts for <1%–4% of reported hydatid cases [1, 5]. It may be postulated that the low prevalence of this form of disease is potentially due to the physical barriers to the hematogenous dissemination of cysts created by hepatic sinusoids and pulmonary capillaries.
In addition, it has been postulated that the higher lactic acid concentration in skeletal muscle and mechanical factors, such as contractile activity, may make encystment less likely [1]. The most common skeletal muscle sites include pelvic, thigh, and paravertebral musculature [6].
Cysts may present in several ways, including a slow-growing lump with variable pain or sudden onset of symptoms due to cyst rupture. Rupture of cysts with release of cyst antigens into muscles causes an inflammatory response that may be complicated by secondary bacterial infection [7]. In addition, involvement of adjacent structures, such as blood vessels, as occurred in our patient, may result in vascular insufficiency [8], and muscle infiltration may produce mechanical limitation of movements [9].
Radiological findings and serology may not always be helpful in primary muscle hydatidosis. Diagnostic images may be confused with other pathological processes, such as malignancy, including sarcoma, or infection. Endovesicular daughter cysts present on imaging of hepatic hydatid disease are not usually seen on ultrasound or CT of skeletal muscle cysts, and calcification is rare [10]. Lesions may appear homogenous and isodense, nonhomogenous and hyperdense, or as a muscle abscess on CT. T1-weighted MRI images show a variable pattern of signal intensity, representing lesions of various ages, but the appearance of lesions is usually not diagnostic of hydatid cysts [11].
Although the majority of patients with viable hydatid cysts have positive results on all forms of serological assay, false-negative serological results are common with senescent or dead cysts and cysts in extrahepatic sites, including skeletal muscle [7]. In Australia, immunoelectrophoresis (arc 5) fails to diagnose infection in up to 20% of patients with proven hydatid disease [12]. Although patients with initial false-negative test results commonly go on to develop positive serological results after treatment, initial serological negativity does not exclude hydatid infection.
Visualization of protoscoleces or germinal membranes in fluid collected by fine-needle aspiration under CT or ultrasound guidance is a safe and effective diagnostic procedure for liver hydatid disease [13, 14]. This procedure has a low complication rate in liver hydatid disease, with infrequent allergic reactions, including urticaria and/or fever [14]. The diagnosis is confirmed by the visualization of hooks, protoscoleces, or germinal membranes in fluid aspirated from liver cysts. If a cyst is infected or degenerative, protoscoleces may be undetectable in the aspirated fluid [14]. However, the usefulness of this procedure in the treatment of skeletal muscle hydatid disease has not been assessed.
The treatment of choice in muscular hydatid disease is excision of the intact cyst and surrounding tissue [1, 8]. The rationale for albendazole therapy after percutaneous aspiration-injection-reaspiration (PAIR) or surgery is to inactivate viable scoleces in the residual cyst and prevent recurrence. A randomized trial comparing albendazole therapy and PAIR demonstrated maximum reduction in cysts treated with concomitant chemotherapy [15]. Recommendations on the timing of commencement of chemotherapy before surgery or PAIR are varied. Adjunctive chemotherapy initiated before surgery is also of benefit for patients with cysts in inaccessible anatomic locations or when there is spillage of cyst contents or rupture into the biliary system [16]. The World Health Organization Working Group on Echinococcus recommends preoperative treatment commencing at least 4 days before surgery or PAIR [17]. Factors that should be considered are the procedure to be done and the size and type of cyst. Thick-walled or large cysts may require prolonged treatment before surgery to achieve a scolicidal effect [18]. Viable protoscoleces obtained intraoperatively and after albendazole therapy may be used to predict recurrence [19]. Albendazole has been shown to significantly decrease intracystic pressure when given for 3 weeks before surgery [20]. Exposure time to the drug appears to be more important than the concentrations achieved. This was illustrated in a prospective randomized study of duration of treatment on cyst viability: After 1 and 3 months of treatment with albendazole for liver cysts, 72% and 94%, respectively, were nonviable [15]. When used alone without surgery, albendazole achieves a cure (cyst disappearance) or improvement, with a reduction in cyst size in up to 50% of cases [17].
Total duration of therapy has varied in studies of PAIR and ranges from 0 to 8 weeks [14, 21–24]. The benefit of treatment with albendazole after surgery is debatable; treatment with albendazole after surgery is likely to have most benefit in conservative procedures in which there is residual cyst tissue, for cysts in inaccessible anatomic locations, or when there is cyst spillage or biliary rupture [16]. The World Health Organization Working Group on Echinococcus recommends 1 month of treatment after surgery [17].
The combination of albendazole and praziquantel has been investigated in vivo in a rat model of hydatid infection. In contrast to monotherapy with either agent, combination treatment produced a significant reduction in both the number and viability of cysts [25, 26]. Albendazole is rapidly converted to an active metabolite, albendazole sulfoxide, which achieves high concentrations in the cyst and is active against both protoscoleces and the germinal membranes [27]. Praziquantel does not penetrate into the mature cyst and, therefore, does not inhibit cyst growth, but it is a highly effective protoscolicidal agent both in vitro and in vivo [28, 29]. The likely role for praziquantel in human hydatidosis may be in preventing encystment of protoscoleces following perioperative spillage [30].
In humans, the use of combination therapy has been reported in the treatment of inoperable spinal [31], pelvic, abdominal, thoracic, and hepatic hydatidosis [32, 33]. In one study, patients with hepatic cysts were treated with a combination of albendazole and praziquantel. These were compared with historical controls treated with albendazole alone. The complete cure rates were higher in the combined group (47.4% vs. 36.4%), although this difference was not statistically significant. In addition, the time to cure was 2–6 months in the combined group, compared with 6–24 months in the albendazole monotherapy group [34]. Therefore, combination therapy may result in a more rapid response than therapy with albendazole alone. Finally, combination therapy was well tolerated, with no increase in adverse events.
Hydatid disease should be considered in the differential diagnosis of cystic lesions of skeletal muscle, because routine diagnostic procedures may not always be helpful.
Surgery with adjunctive chemotherapy is the treatment of choice in the management of complicated hydatid disease to prevent dissemination and recurrence. Albendazole is the most widely used agent, because the role of praziquantel is less clearly defined. Further studies are required to document the benefits of combination therapy over monotherapy.
Acknowledgments
We thank the surgeons of the orthopedic and plastic surgery departments, Royal Melbourne Hospital, for their role in the surgical management of this case.






Comments